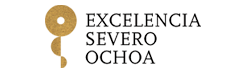Excellence in communication science
Leading journals publish CNIC science
JACC
Spanish scientists provide the first demonstration that triglycerides are a primary risk factor in atherosclerosis
Triglycerides can be as important an indicator of cardiovascular risk as high cholesterol. A study conducted by researchers at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) shows for the first time that hypertriglyceridemia (excess circulating triglycerides) is associated with subclinical atherosclerosis (an accumulation of fats, cholesterol, and other substances within and on the surface of arteries, leading to restricted blood flow) and vascular inflammation in individuals with low-to-moderate cardiovascular risk, even if they have normal circulating concentrations of LDL-C, known as ‘bad’ cholesterol. The results are published in the Journal of American College of Cardiology (JACC).
Until now, triglycerides have been considered a secondary factor in the origin of atherosclerosis, far less important than cholesterol, especially cholesterol bound to low-density lipoproteins (LDL). Indeed, “if LDL-C concentrations are normal, current cardiovascular prevention guidelines do not recommend treatment for high circulating triglyceride concentrations unless the patient has a high cardiovascular risk,” said study first author Dr. Sergio Raposeiras-Roubin.
The new study provides the first demonstration that “in individuals with a low-to-moderate cardiovascular risk according to standard scores (the majority of the population), high circulating levels of triglycerides are associated with a greater risk of developing atherosclerosis, even among people with normal LDL-C.”
The new study forms part of the PESA CNIC-Santander study (Progression and Early detection of Subclinical Atherosclerosis), a major long-term project run by the CNIC in partnership with Santander Bank. The PESA CNIC-Santander study examines the development of atherosclerotic plaques in three arterial territories—the carotids, the abdominal aorta, and the iliofemoral arteries—in an asymptomatic population of Santander Bank employees between the ages of 40 and 54 years. The study, led by CNIC General Director Dr. Valentín Fuster, has demonstrated the high prevalence of subclinical atherosclerosis in the general population, establishing the importance of detecting the disease early in its silent phase.
Moreover, the new JACC article shows that triglyceride levels are associated not only with the presence of atherosclerosis, but also with vascular inflammation.
CNIC Clinical Research Director Dr. Borja Ibáñez explained that this result indicates a strong association between elevated circulating triglyceride levels and the early stages of atherosclerosis, a finding “with important implications for the design of preventive strategies.”
The results indicate that clinical practice guidelines should be modified to emphasize the need to control not only LDL-cholesterol but also triglyceride levels. “The measurement of blood triglycerides is routine, and fortunately there are abundant effective medicines available to ensure appropriate levels,” concluded Dr. Fuster.
The study received funding from the Carlos III Institute of Health and the European Regional Development Fund. Dr. Ibáñez’s research is supported by the European Research Council through the MATRIX project (ERC-COG-2018-ID: 819775).
ACS Nano
CNIC scientists describe a possible disease-causing mechanism in hypertrophic cardiomyopathy
Scientists at the CNIC have described a potential disease-causing mechanism in hypertrophic cardiomyopathy (HCM), the most frequent hereditary disease of the heart. The study, published in the journal CS Nano, provides the first description of an association between this disease and mechanical alterations to a component of the contractile machinery of the heart.
The heart muscle is under constant mechanical stress throughout life as it contracts to pump blood to the body. The laboratory led by Dr. Jorge Alegre-Cebollada investigates how the mechanical properties of the cardiac proteins determine the physiological behavior of this muscle and how alterations to these properties lead to the appearance of diseases like HCM. In this disease, the most frequent hereditary disease affecting the heart, the left ventricle becomes enlarged, and severe manifestations include heart failure and sudden death.
Scientists have known for more than 20 years that HCM is caused by mutations in proteins with a mechanical function in the heart. One of the challenges of cardiovascular genetics is to identify which among the genetic variants found in patients and their families cause disease. Knowing if a mutation is disease-causing or not is important because this information will determine the clinical follow-up of family members and, potentially, their treatment.
The new study, coordinated by Dr. Jorge Alegre-Cebollada, analyzed cardiac myosin-binding protein C (cMyBP-C), the most frequently mutated protein in HCM patients. “A high proportion of mutations in the cMyBP-C gene cause amino-acid changes in the protein; however, the mechanisms by which these mutations cause HCM are not precisely known.”
Dr. Alegre-Cebollada’s group, in close partnership with clinical and molecular researchers in Europe and the US, set up a database of cMyBP-C variants with a clear link to HCM in order to define the molecular defects underlying the disease.
Using bioinformatics and experimental approaches, the research team discovered that around half of these mutations affect the integrity of cMyBP-C messenger RNA (mRNA) or protein. These results have already been accepted for publication in the Journal of Biological Chemistry and have been the subject of a commentary article in the leading medical genetics journal Genetics in Medicine.
While alterations to mRNA or protein integrity could explain the pathogenicity of half the mutations analyzed in the earlier study, Dr. Alegre pointed out that the other half do not cause disease via this route.
Using advanced biophysical techniques based on atomic force microscopy, the team showed that some of the disease-causing mutations in cMyBP-C produce defects in the mechanical properties of the protein that can alter the contractile function of cardiomyocytes in HCM patients.
Identifying the molecular mechanisms underlying HCM is essential for determining which cMyBP-C mutations cause the disease. This knowledge is therefore also crucial for the clinical follow-up and possible treatment of patients and their families, say the authors.
The study was funded by the Ministerio de Ciencia e Innovación, the European Research Area Network on Cardiovascular Diseases (MINOTAUR consortium, through the Instituto de Salud Carlos III), the Comunidad de Madrid, the US National Institutes of Health, the government of the Basque region, the Italian Ministry of Education, Universities and Research, and postdoctoral fellowships from the School of Medicine and the Maternal and Child Health Institute at Stanford University. The research team also included scientists from the Cardiovascular Biomedical Research Network (CIBERCV).

Nature Communications
CNIC scientists identify essential factors for limb formation
Cardiovasculares (CNIC), working in partnership with researchers at the Institut de Recherches Cliniques de Montréal (IRCM) in Canada, have identified Meis transcription factors as essential biomolecules for the formation and antero-posterior patterning of the limbs during embryonic development.
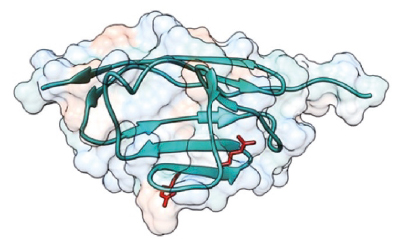
In the study, published in Nature Communications, the research team carried out an in-depth characterization of the Meis family of transcription factors. Genetic deletion of all four family members showed that these proteins are essential for the formation of the limbs during embryonic development. “An embryo that develops in the absence of Meis does not grow limbs,” said study coordinator Miguel Torres, who leads the Genetic Control of Organ Development and Regeneration group at the CNIC.
Embryonic development is a highly complex process involving interactions among a large array of molecules to ensure the correct formation of a specific organ or tissue from a small initial number of cells. The limbs, explained first author Irene Delgado, “start to form as bulges on the flank of the embryo called limb buds. Growth of the limb bud eventually results in the formation of the skeletal components of the limb.”
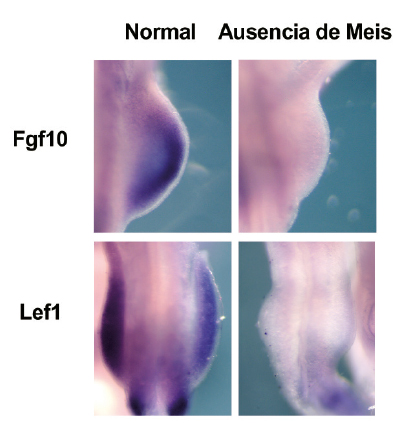
One of the factors that plays a crucial role in the developing limb is a group of transcription factors called Meis. “In a normal embryo, the Meis genes are expressed very early during the formation of the limb buds,” the scientists explained.
In the new study, in-depth molecular characterization of developing mouse embryos revealed that Meis factors initiate a signaling cascade that is essential for limb bud development and involves contributions from Fgf10 and Lef1. “Our results identify roles for Meis transcription factors in the developing limb and reveal their participation in essential pathways for limb development. During ealry limb bud formation, Meis transcription factors are essential for inducing the expression of Fgf10 and Lef1.”
An embryo that lacks Meis genes is unable to grow limbs, but the presence of just one of these four genes (a single allele) “is enough to initiate limb development and also reveals other functions of Meis, such as its importance for the formation of the proximal limb structures (pelvis and femur) and for antero-posterior limb patterning,” said Miguel Torres.
Nevertheless, the pelvis and femur of embryos with a single Meis allele are smaller than those of a normal embryo. Moreover, added Delgado, “These embryos have defects in, or simply lack, posterior skeletal elements such as the fibula and posterior digits.”
The authors further demonstrated that the molecular basis for these defects is failed initiation of the expression of the Sonic Hedgehog gene, which is essential for antero-posterior limb patterning.
JACC
A blood sugar biomarker identifies patients with atherosclerosis and a risk of cardiovascular events
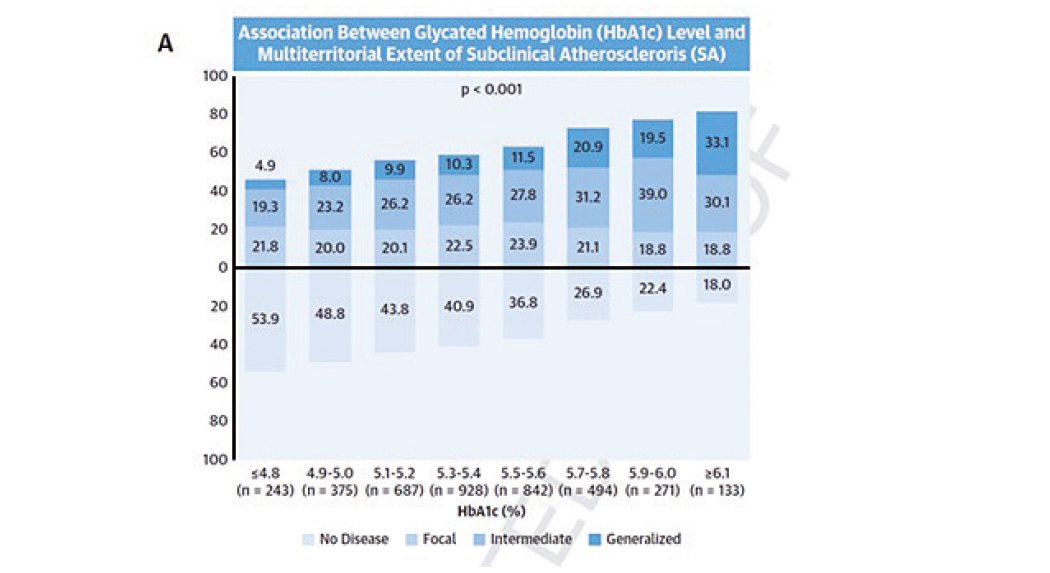
The routine use of the glycosylated hemoglobin test to track blood sugar levels in the general population can identify individuals with more advanced atherosclerotic disease. Currently used in the diagnosis and management of diabetes, glycosylated hemoglobin can provide a useful estimate of atherosclerotic disease, and therefore of cardiovascular risk, in individuals without diabetes with or without possible prediabetes.
The advance heralded by the CNIC study is the use of this blood-sugar measure in apparently healthy middle-aged individuals who do not have diabetes mellitus but with or without possible prediabetes.
When used in combination with traditional risk factors (hypertension, dyslipidemia, and smoking), the glycosylated hemoglobin test more accurately distinguishes between people at high and low risk of atherosclerotic disease.
The study, published in the Journal of American College of Cardiology (JACC), proposes that, because glycosylated hemoglobin levels can easily be reduced through lifestyle changes, the test should be at the front line of risk reduction strategies
The glycosylated hemoglobin diagnostic test is cheap, accessible, and widely used in daily clinical practice, explained Dr. Xavier Rosselló, CNIC scientist and cardiologist at Hospital Universitario Son Espases in Palma de Mallorca. The test can therefore be put to immediate use to calculate the degree of subclinical atherosclerosis in the general population.
The study forms part of the collaborative PESA-CNIC- Santander project, which is led by Dr. Fuster. Begun in 2010 and recently extended until 2030, the PESA-CNIC-Santander study is one of the most important cardiovascular prevention studies in the world.
Atherosclerosis is usually only detected at advanced stages, after it has already triggered a cardiovascular event such as a myocardial infarction or stroke. Events of this type severely worsen patient prognosis, and the early detection of atherosclerosis.
The new CNIC finding provides a simple way to increase the accuracy of cardiovascular risk ranking in individuals without diabetes with or without prediabetes .
The authors conclude that “this study establishes glycosylated hemoglobin as a mass-use biomarker because its greatest benefit is in individuals at low cardiovascular risk, who form the immense majority of the general population and account for most cardiovascular deaths in absolute terms.”
The study was funded by the Instituto de Salud Carlos III (ISCIII, PI15/ 02019, PI17/00590, PI20/00819) and the European Regional Development Fund (ERDF) “A way to make Europe” and included scientists from the CIBER de Enfermedades CardioVasculares (CiberCV) research network.
NEJM
The first blood biomarker to distinguish between myocarditis and acute myocardial infarction
Scientists at the CNIC have identified the first blood biomarker for myocarditis, a cardiac disease that is often misdiagnosed as myocardial infarction. Nevertheless, the diagnosis of myocarditis continues to be challenging in clinical practice.
The study, led by Dr. Pilar Martín and published in The New England Journal of Medicine, has detected the presence of the human homolog of micro RNA miR-721 in the blood of myocarditis patients.
CNIC General Director Dr. Valentín Fuster emphasizes that these results of paramount importance because they establish the first validated blood marker with high sensitivity and specifity (>90%) for myocarditis. This will allow clinicians to distinguish between this disease and other cardiomyopathies like acute myocardial infarction, myocardial infarction with nonobstructive coronary arteries (MINOCA), and other inflammatory diseases with an autoimmune origin.
“Our finding has great potential as a valuable clinical tool for the precise and noninvasive diagnosis of myocarditis from small drops of blood,” says Dr. Martín, whose project is funded by a Fundación BBVA Beca Leonardo award.
The diagnosis of myocarditis is challenging, and the availability of a sensitive and specific marker of acute myocardial inflammation could have a major clinical impact, improving the diagnosis of myocarditis both generally and particularly in its early phases.
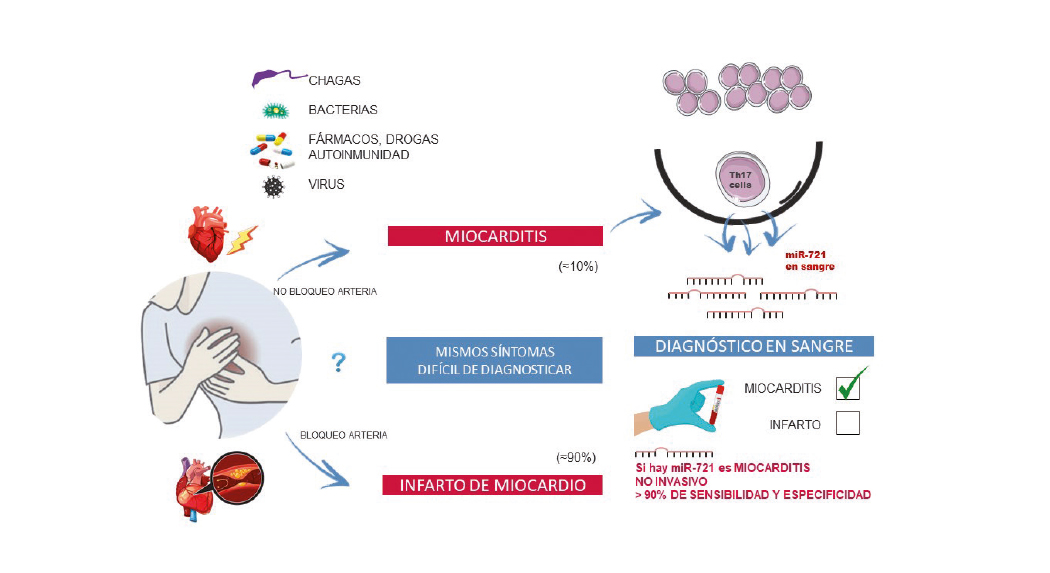
Myocarditis is an inflammatory disease of the heart caused by infection, toxins, drugs, or autoimmune disorders. If untreated, myocarditis can progress to potentially fatal dilated cardiomyopathy, requiring heart transplant.
The prevalence of myocarditis remains uncertain because it is often difficult to achieve a confirmed diagnosis.
Myocarditis is usually diagnosed after coronary angiography or computed tomography scans have discarded coronary artery disease, followed by confirmation of the diagnosis by magnetic resonance imaging (MRI).
However, not all centers have access to MRI technology, and the current gold standard for myocarditis diagnosis is endomyocardial biopsy, an invasive procedure normally reserved for severe cases. There is thus a pressing clinical need for the development of reliable and accessible tools for the early diagnosis of acute myocarditis.
Moreover, myocarditis is a side effect that, although very rare, is potentially serious in cancer patients who are receiving treatment with immunotherapy drugs called “immune checkpoint inhibitors”.
There are currently no specific markers for the diagnosis of patients susceptible to developing myocarditis during cancer immunotherapy.
The researchers are currently designing studies to evaluate the potential of the biomarker as a predictor of short-term and long-term risk, the persistence of myocardial inflammation, and the risk of relapse, clinical progression, and adverse ventricular remodeling.
The CNIC is the sole owner of a patent related to the biomarker and its use for the diagnosis of miocarditis. The CNIC is now exploring licensing agreements with industrial partners to develop and commercialize this technology in order to make it available for clinical use.
The study received funding from the Ministerio de Ciencia e Innovación(MICINN) through the Instituto de Salud Carlos III (ISCIII)-Fondo de Investigación Sanitaria; the CIBERCV; the Comunidad de Madrid; a Fundación BBVA Beca Leonardo award; Fundació La Marató TV3; and European Research Council grants ERC-2011-AdG 294340-GENTRIS to F.S-M. and ERC-2018-CoG 819775-MATRIX to B.I.

JITC
CNIC scientists discover a new strategy to improve cancer immunotherapy
Scientists at the CNIC have designed a new strategy to potentiate immunotherapy, the treatment that has revolutionized cancer management. In the study, published in the Journal for Immunotherapy of Cancer, the team led by Dr. David Sancho identifies a mechanism through which dead tumor cells stall the response of the immune system, reducing the antitumor capacity of immune cells that attack cancers.
The scientists improved the efficacy of cancer immunotherapy through an approach that combines blocking antibodies with cytokines or chemotherapy treatments that promote the development of dendritic cells. The team believe that this approach can be transferred to the clinic to optimize cancer immunotherapy.
Immunotherapy consists of reprogramming the immune system to recognize and eliminate tumor cells.
Unfortunately, not all patients benefit from this approach, because many cancers develop mechanisms to evade the immune system. One of these mechanisms consists of impeding the migration of immune cells to the tumor microenvironment. “The immune system has the ability to enter the tumor and eliminate it, thus improving patient prognosis. However, this does not always happen, and the aim of this project was to strengthen this capacity,” explained study coordinator Dr. David Sancho, who heads the Immunobiology laboratory at the CNIC.
The study shines a light on the mechanisms that promote correct infiltration of the tumor by antitumor immune cells and proposes a new strategy to potentiate this infiltration in antitumor immunotherapy.
The type of immune cells that enter the tumor has a major influence on cancer patient survival. Survival is higher after infiltration by CD8 T cells, which eliminate tumor cells, and by dendritic cell subtypes that attract and activate CD8 T cells. Scientists in the field have therefore focused their efforts on developing methods to increase the numbers of these cells in the tumor microenvironment.
Immune cells have an extensive repertoire of receptors through which they interact with their environment, allowing them to recognize pathogens and also detect tissue damage. Dr. Sancho’s team recently showed that the detection of dead cells by the receptor DNGR-1, expressed on dendritic cells, prevents excessive inflammation. In cancer, this action can cause more harm than good. “High immune-cell infiltration of the tumor microenvironment will promote tumor elimination by the immune system,” said Dr. Sancho.
Dendritic cells form part of the innate immune system, the body’s first line of defense. The presence dendritic cells in the tumor environment “goes a long way to ensuring an immune response against the tumor that will improve patient prognosis,” said Dr. Fresno.
In the new study, the scientists found that the recognition of dead tumor cells through DNGR-1 expressed on dendritic cells prevents further infiltration of both dendritic cells and the tumor-eliminating CD8 T cells, thus preventing the immune system from attacking the tumor. The researchers managed to increase the efficiency of antitumor immunotherapy by using blocking antibodies in combination with cytokines or chemotherapy to promote dendritic cell development.
Immunotherapy is the reprogramming of the immune system to recognize and eliminate tumor cells; however, this approach does not work in all patients because many cancers develop mechanisms to evade the immune response.
The research shows that the antitumor activity of dendritic cells can be promoted through targeted interventions. The first of these is the administration of the cytokine Flt3L, and the second is antibody blockade of DNGR-1. Flt3L acts as a ‘stimulant’, increasing the numbers of dendritic cells and promoting their entry into the tumor microenvironment. The team also found that circulating levels of Flt3L can be increased by stimulating the body’s own production of the cytokine with specific chemotherapy treatments.
The new study shines a light on some of the mechanisms that promote correct tumor infiltration by antitumor immune cells and proposes a new strategy to potentiate this infiltration and antitumor immunotherapy. The study thus identifies a new tool for combating cancer by strengthening the body’s own defense.
The study was funded by the Fundación “la Caixa”, the Asociación Española contra el Cáncer (AECC), the National Institute for Health Research Manchester Biomedical Research Center, the European Research Council (ERC-2016-Consolidator Grant 725091), the European Commission (635122-PROCROP H2020), the Ministerio de Ciencia, the Agencia Estatal de Investigación (AEI), the European Regional Development Fund (ERDF) (SAF2016-79040-R), the IMMUNOTHERCAN de la Comunidad de Madrid, a FIS-Instituto de Salud Carlos III grant, the Fundación Acteria, Atresmedia (Constantes y Vitales), and Fundació La Marató de TV3.

Nature Communications
Inhibition of proteins activated by nitric oxide reverses aortic aneurysm in Marfan syndrome
Scientists at the CNIC and Centro de Biología Molecular Severo Ochoa (CBM-CSIC-UAM) have discovered that the nitric oxide (NO) pathway is overactivated in the aortas of mice and patients with Marfan Syndrome and that the activity of this pathway causes the aortic aneurysms that characterize this disease.
The results of the study, published in Nature Communications, reveal the essential role played by NO in Marfan Syndrome aortic disease and identify new therapeutic targets and markers of NO pathway activation that could be used to monitor disease status and progression.
Aortic aneurysm (AA) is a progressive dilatation and weakening of the aortic wall. AA can be harmless, but in some patients can lead to dissection (rupture) of the aorta, resulting in death.
The study, funded by the Fundación “la Caixa”, identifies new biomarkers associated with this syndrome that have the potential to improve the clinical treatment and prognosis of Marfan Syndrome patient’s syndrome.
Marfan Syndrome is a hereditary disease that affects connective tissues, which are the fibrous structures that bind and anchor all the organs and tissues in the body. Marfan Syndrome principally affects the skeleton, the eyes, the heart, and the blood vessels. A particularly common disease manifestation is thoracic aortic aneurysm and dissection (TAAD). Aortic dissection accounts for more than 90% of deaths associated with Marfan Syndrome.
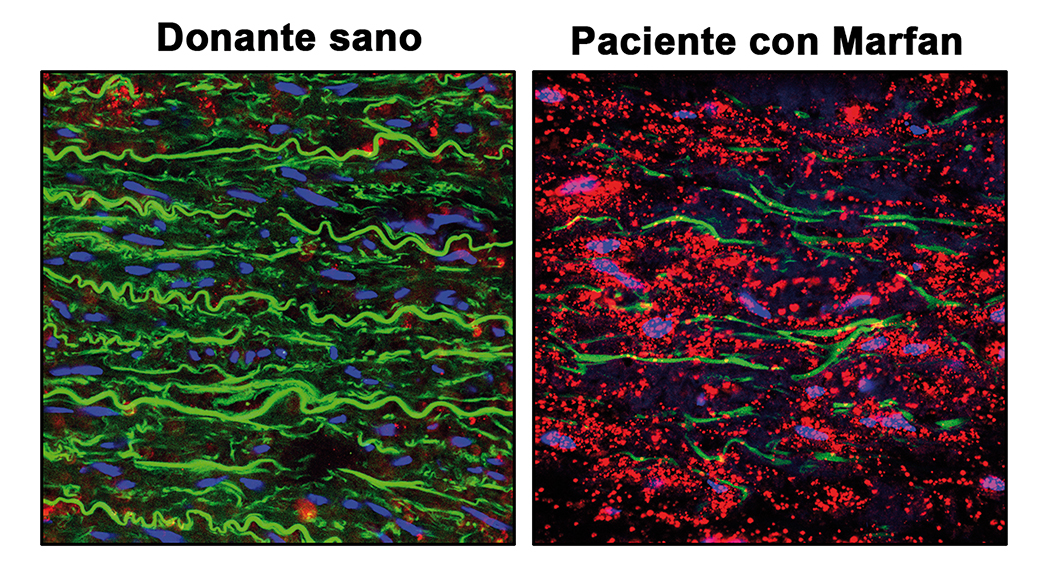
Current treatments for Marfan Syndrome are aimed at reducing blood pressure on the artery wall, but do not prevent its deterioration. The only effective intervention for the aortic disease in Marfan Syndrome is surgery.
The researchers therefore recognize “the urgent need to identify new targets for the development of pharmacological treatments for TAAD in Marfan Syndrome.”
The researchers have demonstrated that silencing or inhibiting the activity of these proteins completely reverses the aortic disease in a mouse model of Marfan Syndrome .
The study reveals the essential role played by NO in Marfan Syndrome aortic disease. “We previously detected high expression of a protein with a high capacity for NO production in the aortas of Marfan Syndrome patients and an animal model of the disease, and we therefore undertook an in-depth investigation of the role of NO in the associated aortic disease,” explained.
“We observed that treatment of healthy mice with supra-pharmacological doses of an NO donor induced TAAD similar to that seen in Marfan mice. The NO donor treatment also reproduced the degeneration of the aortic wall, an essential step in the development of TAAD,” added Dr. Redondo. “Through these experiments, we showed that elevated production of NO is necessary and sufficient for the development of TAAD in Marfan Syndrome.”
Given this important role of NO in the development of TAAD, the researchers decided to focus on the enzymes soluble guanylate cyclase (sGC) and type I cGMP-dependent protein kinase (PRKG1), two NO-regulated proteins. “Our analysis detected elevated activities of both sGC and PRKG1 in samples from mice and patients with Marfan Syndrome,” said Dr. Redondo.
“We were able to completely reverse the aortic disease in Marfan mice by treating them with inhibitors of these two proteins or by genetically silencing the expression of Prkg1, demonstrating that the NO-sGC-PRKG1 pathway mediates the development of TAAD in Marfan Syndrome,” added Dr. Campanero.
Given the need for new pharmacological treatments for Marfan Syndrome aortic disease, “the results of this study open the way to the use of sGC and PRKG1 inhibitors in preclinical and clinical trials for this syndrome and possibly other aortic diseases,” said Dr. Redondo.
The research team also explored possible “footprints” left by high NO levels in the blood.
“This discovery has important implications for patients with this syndrome, because these molecules could be used as biomarkers for disease monitoring, and we are now studying their potential as prognostic indicators,” explained Dr. Redondo.
The study was funding by the Fundación “la Caixa” through the CaixaResearch Call for Health Projects with 500.000 euros, the Ministerio de Ciencia, Investigación y Universidades, Comunidad de Madrid, el CSIC, the Pro-CNIC Foundation, Marfan Foundation, the Fundación La Marató y the CIBER de cardiovascular (CIBER-CV) Instituto de Salud Carlos III.

JACC
CNIC and Philips develop ultrafast cardiac magnetic resonance technology that analyzes the heart in less than 1 minute
Scientists at the CNIC and Philips have developed a revolutionary technology that can perform cardiac magnetic resonance (CMR) scans in under a minute. ESSOS (Enhanced SENSE by Static Outer volume Subtraction) allows precise assessment of heart anatomy and function, as well as reducing healthcare costs and increasing patient comfort. The new methodology has been tested on more than 100 patients with diverse heart conditions. The results have just been published in JACC: Cardiovascular Imaging, the world-leading journal in the field of cardiac imaging.
CMR provides a noninvasive and radiation-free method for exploring the heart and is the ideal technique for studying heart anatomy, function, and even cell composition. Although most hospitals have magnetic resonance scanners, these are not often used for heart studies because a complete CMR study takes so long. Study first author Dr. Sandra Gómez-Talavera, a CNIC investigator and cardiologist at Fundación Jiménez Díaz University Hospital, explained that “a complete CMR study takes 45-60 minutes, and many patients don’t go through with it because remaining in the scanner for this long is too uncomfortable.”
In addition, hospital magnetic resonance scanners are needed for other studies, limiting their availability for long-duration cardiac studies.
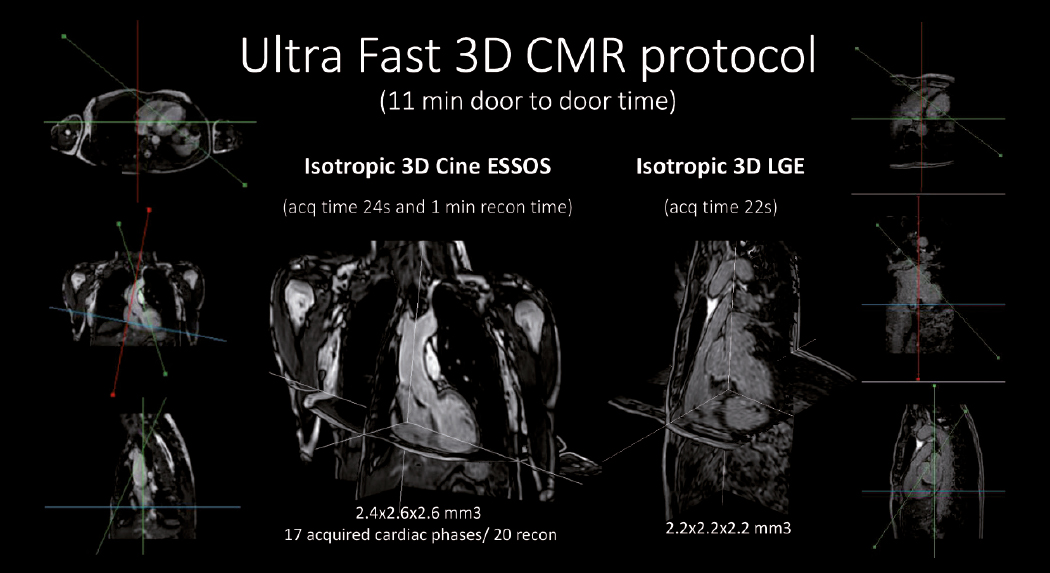
To overcome these obstacles to CMR, CNIC scientists working in partnership with Philips have developed a technique for accelerated CMR acquisition. The technique, explained Dr. Gómez-Talavera, “allows the study of the anatomy and function (motility) of the heart muscle, as well as infarcted and fibrotic tissue. The method can be used to study the whole thoracic cavity in 3D, with algorithms used to focus exclusively on the heart and major vessels (the mobile elements), reducing the scan time.”
The technique, called ESSOS (Enhanced SENSE by Static Outer volume Subtraction), has been tested on more than 100 patients with diverse heart conditions.
“We have demonstrated in a large group of patients that CMR with this technology yields the same information as the standard technique, but for less than 10% of the patient scan time,” said Dr. Borja Ibáñez, CNIC Clinical Research Director, a cardiologist at Fundación Jiménez Díaz University Hospital, and CIBERCV group leader. Dr. Ibáñez is one of the two lead authors on the study.
ESSOS is protected by a patent held jointly by the CNIC and Philips and is the fruit of almost 10 years’ collaboration. The research team believe that this new technology will revolutionize cardiac imaging.
ESSOS allows images to be obtained 34 times faster than with the standard technology in current use. “All the information needed to understand heart form and function can be acquired in a little over 20 seconds,” indicated Sánchez-González, adding that “a further 20-second acquisition is all that is needed to detect infarction or fibrosis. This brings the scan to an end, in less than 1 minute.”
A key advantage of the technology is that it can be used with the magnetic resonance scanners already installed in hospital .
The research was funded by the Instituto de Salud Carlos III through a FIS technology development grant, a Spanish Society of Cardiology Translational Research award, the European Research Council (ERC), and the Comunidad de Madrid (Red Madrileña de Nanomedicina en Imagen Molecular).
JACC
CNIC researchers explain how high blood pressure, the most important cause of disease worldwide, accelerates atherosclerosis
High blood pressure, the most important cause of disease worldwide, accelerates atherosclerosis but the mechanism is unknown. Using gene modified minipigs, researchers from the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Aarhus University (Denmark), demonstrate that high blood pressure alters the structure of arteries leading to more accumulation of LDL cholesterol and faster development of atherosclerosis. The study has been published in the Journal of the American College of Cardiology (JACC).
Blood pressure-lowering drugs are routinely used to prevent the development of atherosclerosis and heart disease, but the mechanism of this effect is still unknown. People suffering from high blood pressure (hypertension) often have accompanying changes in the hormones that control blood pressure and it has been unclear whether the pressure itself or the hormonal changes are the driver of accelerated atherosclerosis.
To investigate this, researchers from the CNIC and Aarhus University analyzed the development of atherosclerosis in minipigs that were genetically engineered to have high blood cholesterol and develop atherosclerosis.
Minipigs have arteries that are very similar in structure to human arteries and like humans they develop atherosclerosis in the heart when exposed to high blood cholesterol, Dr. Jacob Fog Bentzon comments, coordinator of the study published in JACC. As is also the case in humans, the development of the early stages of the disease is asymptomatic and therefore experiments on atherosclerosis can be conducted in minipigs with high animal welfare.
By manipulating blood pressure in the pigs and by analyzing the effects on arteries in the heart, the researchers found that the direct forces of pressure on arteries leads to structural changes that facilitate the development of atherosclerosis. “Arteries become denser and allow less passage of molecules from the blood. This includes the LDL particles that carry blood cholesterol, which instead accumulate in the innermost layer of arteries, where they drive the development of atherosclerosis”, Dr. Jacob Fog Bentzon explains.
This finding uncovers an intimate relationship between the most important risk factors for atherosclerosis, LDL cholesterol and high blood pressure. While it has been known for decades that accumulation of LDL particles in arteries lead to atherosclerosis, the new research shows that high blood pressure accelerates the accumulation of LDL. Therefore, high blood pressure aggravates the effect of having high LDL cholesterol in the blood.
The new insight supports the need to keep both LDL cholesterol and blood pressure low throughout life by healthy diet choices, weight control, exercise, and, when needed, by drug therapy. “It could also pave the way for the development of more effective therapies to offset the detrimental effects of hypertension on atherosclerosis”, the researchers conclude.
The research was a collaboration among the Experimental Pathology of Atherosclerosis at CNIC, the Cardiovascular Proteomics groups at CNIC, CIBER de Enfermedades Cardiovasculares, and the Atherosclerosis Research Unit at Aarhus University in Denmark.
The work at CNIC was funded by the Ministerio de Economia, Industria y Competividad with cofunding from the Fondo Europeo de Desarrollo Regional (SAF2016-75580-R and PGC2018- 097019-B-I00), the Instituto de Salud Carlos III-Fondo de Investigación Sanitaria (IPT17/0019–ISCIII-SGEFI/ERDF, ProteoRed), the Fundació la Marató de TV3 (grant 122/C/2015) and “la Caixa” Foundation (project code HR17-00247).

JACC
CNIC scientists identify mutations acquired by blood cells that accelerate heart failure progression
A team of scientists at the CNI) and the Hospital Universitario Virgen de la Arrixaca in Murcia has discovered that Clonal hematopoiesis increase the risk of rapidly progressing heart failure, one of the chief causes of death in the world.
The adult human body produces hundreds of billions of blood cells every day. This essential process unavoidably leads to the appearance of mutations in the DNA of the progenitor cells. These are known as somatic mutations because they are acquired, not inherited. While most of these mutations are innocuous, occasionally a mutation gives affected cells a competitive advantage that allows them to expand progressively, generating clonal populations of blood cells. This phenomenon is known as clonal hematopoiesis.
Clonal hematopoiesis is linked to aging, because over time there is an increasing chance that a culprit mutation will be produced, explained Dr. José Javier Fuster, coordinator of the study published in the Journal of the American College of Cardiology (JACC).
“Recent studies showed that people with clonal hematopoiesis have a higher risk of developing hematological cancers and dying. Curiously, however, the death of these patients is often due not to the cancer, but to cardiovascular causes.”
“There is established evidence linking clonal hematopoiesis to an increased risk of atherosclerosis, the underlying cause of most heart attacks and a high proportion of strokes,” commented Dr. Domingo Pascual-Figal, an external investigator at the CNIC and a cardiologist at the Hospital Universitario Virgen de la Arrixaca in Murcia.

The study shows that clonal hematopoiesis is an important pathological process that accelerates and aggravates the clinical progression of heart failure, independently of the presence of atherosclerosis
In the new study, which included input from the CNIC Genomics and Bioinformatics Units and investigators at Hospital Universitari Germans Trias i Pujol in Badalona (Barcelona), the research team analyzed how the presence of mutations linked to clonal hematopoiesis affects the clinical progression of patients with ischemic or non-ischemic heart failure.
Commenting on the results, Dr. Fuster said that, independently of the origin of heart failure, “the presence of these mutant blood-cell clones aggravates disease progression and worsens prognosis.”
Dr. Pascual-Figal explained that the specific study finding was that “clones with mutations in 2 genes frequently linked to clonal hematopoiesis, TET2 and DNMT3A, were associated with a higher risk of heart–failure-related hospitalization and death.”
For the researchers, these findings “demonstrate the importance of clonal hematopoiesis as a pathogenic process that accelerates and aggravates heart failure progression, independently of the presence of atherosclerosis.”
The authors conclude that their study supports the emerging idea that “clonal hematopoiesis represents a new cardiovascular risk factor and an important link between aging and cardiovascular disease.” The results, moreover, “open the way to the development of personalized therapies for patients with these somatic mutations, with the aim of preventing heart failure progression.”
The study was funded by a Beca Leonardo para Investigadores y Creadores Culturales (2019) from the Fundación BBVA, the Carlos III Institute of Health, the Spanish Ministry of Science and Innovation, and the Fundación Séneca de Ciencia y Tecnología de la Región de Murcia. José Javier Fuster is a member of the Transatlantic Network on “Clonal hematopoiesis and atherosclerosis” funded by the Leducq Foundation.
JACC
Scientists uncover early links between cardiovascular risk and brain metabolism
The links between cardiovascular disease and cognitive impairment begin years before the appearance of the first clinical symptoms of either condition. In a study carried out at the CNIC in partnership with Santander Bank and neuroimaging experts at the Barcelonaβeta Brain Research Center (BBRC, the research center of the Fundación Pasqual Maragall), the investigators have identified a link between brain metabolism, cardiovascular risk, and atherosclerosis during middle age, years before the first appearance of symptoms.
The report, published in the Journal of the American College of Cardiology (JACC), is important because it suggests that intervention in a modifiable condition (cardiovascular disease) could prevent the development of dementia, a disease for which there is currently no cure.
Dr. Valentín Fuster, CNIC and Mount Sinai Heart General Director, Physician-in-Chief of the Mount Sinai Hospital, and a lead author on the study, explained that “although everybody knows about the importance of caring for ourselves and controlling cardiovascular risk factors in order to avoid a heart attack, the association of these same risk factors with cognitive decline may increase awareness of the need to acquire healthy habits from the earliest stages of life.”
Moreover, the results provide yet more support for the importance of implementing primary cardiovascular prevention strategies in middle age as a valuable therapeutic approach to slowing or even halting brain alterations that could contribute to future cognitive decline.
The advanced stages of vascular disease and dementia often occur together, but until now this association has not been documented at earlier stages. The CNIC-coordinated study, led by Dr. Marta Cortés Canteli, shows that in middle age, years before any clinical signs appear, atherosclerosis and cardiovascular risk factors already show an association with low metabolism in brain regions implicated in the future development of dementia, especially Alzheimer disease.
Using advanced imaging by positron emission tomography (PET), the research team quantified brain metabolism in more than 500 participants in the PESA-CNIC-Santander study. The participants had an average age of 50 years and no symptoms, but already had evidence of atherosclerosis in their arteries.
PESA-CNIC-Santander, directed by Dr. Valentín Fuster, is a prospective study of more than 4000 asymptomatic middle-aged participants who have been exhaustively assessed for the presence and progression of subclinical atherosclerosis since 2010.
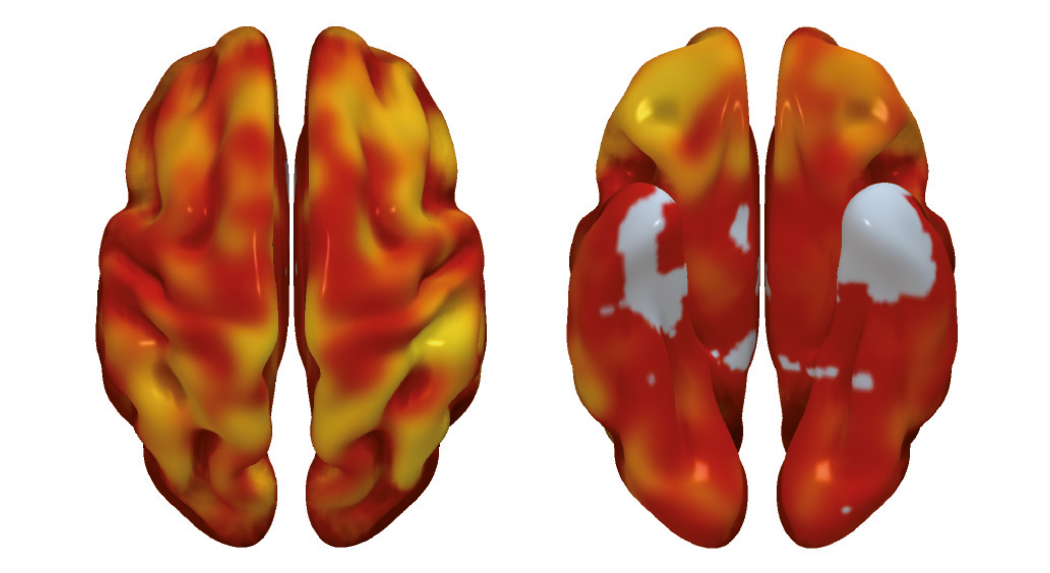
“We found that a higher cardiovascular risk in apparently healthy middle-aged individuals was associated with lower brain metabolism in parietotemporal regions involved in spatial and semantic memory and various types of learning,” said Dr. Cortés Canteli. Dr. Juan Domingo Gispert, head of the Neuroimaging group at the BBRC, noted that “the brain areas showing low metabolism in participants with higher cardiovascular risk are the same areas affected in Alzheimer disease, suggesting that these individuals may have higher than normal vulnerability to this disease.”
The study is the largest of its type to date in a healthy middle-aged population and could signal a paradigm change in the understanding of the links between vascular and brain disease, say the authors.
Among the modifiable cardiovascular risk factors most closely associated with a reduction in brain metabolism, the investigators saw the biggest effect with hypertension. “We found that the same risk factors that damage the heart and the large arteries, and especially hypertension, are closely linked to the decline in brain metabolism years before the appearance of symptoms,” said Dr. Fuster.
The research team also found that a higher number of plaques in the carotid arteries, which carry blood to the brain, was associated with lower brain metabolism in areas of the limbic system and the parietal lobe, both of which are intimately linked to the development of Alzheimer disease.
“The next step will be to determine whether individuals with subclinical atherosclerosis in the carotid arteries and low brain metabolism at the age of 50 go on to experience cognitive decline 10 years later,” said Dr. Cortés Canteli.
These results will be a major stimulus for the implementation of early intervention strategies to reduce the incidence of cognitive decline in old age.
The PESA study is cofinanced equally by the CNIC and Santander Bank. PESA receives additional funding from the Instituto de Salud Carlos III, Madrid, Spain (ISCIII, PI15/02019, PI17/00590 & PI20/00819), the European Regional Development Fund (ERDF - A Way to Build Europe), and the European Social Fund (ESF - Investing in Your Future ). The CNIC is supported by the ISCIII, the MCIN and the Pro-CNIC Foundation. The BBRC is financed mainly by the Fundación “la Caixa”, the EU/EFPIA (European Federation of Pharmaceutical Industries and Associations) Innovative Medicines Initiative Joint Undertaking EPAD, and the Innovative Medicines Initiative 2 Joint Undertaking. This joint project was supported by the European Union Horizon 2020 research and innovation programme and the EFPIA.
Science Advances
Scientists identify a mechanism through which dendritic cells improve their antiviral and immune-activation abilities
Researchers at the CNIC led by Professor Francisco Sánchez-Madrid have found that dendritic cells, which initiate specific immune responses, can reprogram their genes to improve their immune response. The results of the study, funded by Fundación “la Caixa” and published in Science Advances, could have important applications in the development of new vaccination and immunotherapy strategies.
Dendritic cells are professional antigen-presenting cells that initiate adaptive or specific immune responses. As described by the research team, “dendritic cells capture possible pathogenic agents in different tissues and entry sites, process their components, and transport them to lymph nodes. Here, they establish communication with T lymphocytes through the formation of a specialized structure called the immune synapse. The immune synapse allows the dendritic cell to present processed components of the infectious agent to a T cell, so that they can be recognized and initiate a specific T cell immune response.”
Until now, activation of T lymphocytes was thought to be dendritic cells’ main function. However, Prof. Francisco Sánchez-Madrid’s group, working together with the group led by Dr. Almudena R. Ramiro, have discovered that the dendritic cell also receives information from the T cell via the immune synapse. “The T cell sends instructions that induce a change in the dendritic cell’s gene-expression program, promoting the expression of genes related to motility, antiviral responses, and secretion and thereby increasing the dendritic cell’s capacity to generate protective anti-pathogen immune responses,” explained Sánchez-Madrid.
“This study describes how gene-expression changes are accompanied by changes in epigenetic marks on DNA. These epigenetic marks in turn produce transient changes in specific genes that promote or hinder their expression,” explained first authors Irene Fernández Delgado and Diego Calzada Fraile.

The research team found that, after participating in an immune synapse, dendritic cells migrate more efficiently to lymph nodes, where most processes involved in the activation of specific or adaptive immune responses take place.
The new study, carried out in close partnership with the CNIC Bioinformatics Unit (directed by Fátima Sánchez- Cabo) and Genomic Unit (Ana Dopazo), describes a new mechanism that explains how dendritic cells improve their antiviral and immune-activation abilities.
The researchers conclude that their study shows that dendritic cells, responsible for initiating specific immune responses, reprogram their genes through altered epigenetic DNA marks after interacting with a cognate T cell. “These changes improve their motility, so that they arrive sooner at immune response activation sites, representing a new mechanism for potentiating the immune response.”
The results also have potential applications in the development of new vaccination and immune therapy strategies. For example, the described mechanism could be used to generate super-migratory post-synaptic dendritic cells able to induce stronger and more effective immune responses.
The study was supported by funding from Fundación “la Caixa” through Health Research Projects call HR17-00016 and an INPhINIT ‘Retaining’ doctoral project grant”.