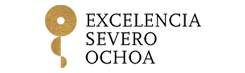EXCELENCIA EN DIVULGACIÓN CIENTÍFICA
Leading journals publish CNIC science
NATURE
CNIC scientists discover a cell behavior pattern that predicts cardiovascular disease

Scientists led by Dr. Andrés Hidalgo at the CNIC have discovered that circulating neutrophils—a type of immune cell—acquire different behavior patterns during inflammatory processes. The study, published in Nature, identifies a harmful neutrophil behavior associated with cardiovascular disease.
The study provides important information that could lead to new treatments to minimize the consequences of myocardial infarction.
Neutrophils are immune cells that form the first line of defense in the body but that can also damage healthy cells, including cells in the cardiovascular system.
The scientists designed a highly novel computational system that allowed them to analyze how cells behave in vessels through simple measures of changes in size, shape, and movement. This analysis identified three neutrophil behavior patterns during inflammatory processes but showed that only one of them, characterized by large size and proximity to the vessel wall, is linked to cardiovascular injury.
Combining this computational system with massive genetic analysis in animal models, the authors were able to identify the molecules responsible for the harmful neutrophil behavior.
The team found that the cause of this pathological behavior is a single molecule, Fgr. This discovery provides the key to selecting highly effective drugs able to prevent inflammation and cell death after a myocardial infarction. “The idea now is to continue with further tests and analysis needed to convert this into a clinical treatment for patients,” said first author Georgiana Crainiciuc.
The scientists believe that the study signals a major advance not only toward improved treatment of cardiovascular disease, but also in the methodology for analyzing immune cells.
“Our model is unique because it allows the identification of cells not from their genetic profile but from their activity during a disease. This is a completely new approach to the study of immune processes that exploits the dynamism of the disease state to generate new information,” explained co-first author Dr. Miguel Palomino-Segura.
“The key to this approach is the ability of neutrophils to change their shape, activity, and capacity to migrate in a matter of seconds. These rapid changes can only be captured under the microscope,” said Dr. Hidalgo.
The scientists made the discovery using high resolution intravital microscopy, which allows the visualization of cells within the blood capillaries of live animals
To extract the full potential of these images, the research team collaborated with engineers at Universidad Carlos III de Madrid, who developed new computer vision techniques for taking measurements in living tissues.
The authors hope that this new methodology will find application in other scientific arenas. “The idea now is to apply this technology in other settings, such as infection or cancer, in which immune cells also play a critical role in disease progression,” said Palomino-Segura.
Collaborators on this project include researchers at Fundación Vithas, Universidad de Castilla-La Mancha, the Singapore Agency for Science, Technology and Research (A*STAR), and Harvard and Baylor Universities in the USA.
The study received support from the following sources: Ministerio de Ciencia e Innovación; Fundación “la Caixa”, Fundación Leducq, FET-OPEN European Commission, Federation of European Biochemical Societies and EMBO ALTF.
Nature Cardiovascular Research
Mutations acquired by blood cells are an indicator of cardiovascular risk
Scientists at the CNIC and Columbia University, New York have published a review article in Nature Cardiovascular Research examining the role in cardiovascular disease of acquired mutations linked to clonal hematopoiesis.
In the article, CNIC scientist José Javier Fuster and Dr. Alan Tall of Columbia University review current knowledge on the role of clonal hematopoiesis in cardiovascular disease, highlighting areas where further research promises to transfer recent advances into tools for the prevention or treatment of cardiovascular disease.
Numerous clinical studies in recent years have demonstrated that acquired mutations linked to clonal hematopoiesis are associated with an elevated risk of multiple cardiovascular diseases, including myocardial infarction, stroke, and heart failure. In recent years, cardiovascular researchers have debated the possibility that these mutations, which are also linked to a heightened risk of hematological cancer, contribute directly to the development of disease.
Work in several laboratories, including those led by Drs. Fuster and Tall, has shown that some mutations linked to clonal hematopoiesis accelerate the development of cardiovascular disease and aggravate inflammatory responses.
“A deeper understanding of these mechanisms could, in the future, lead to personalized strategies for cardiovascular prevention, aimed specifically at countering the proinflammatory effects of these mutations,” said José Javier Fuster.
Despite the availability of effective treatments for traditional cardiovascular risk factors, cardiovascular disease continues to be the leading cause of death, explained Dr. Fuster.
Scientists have known for some years that inflammation is a central factor in cardiovascular disease, but this has not led to the general use of anti-inflammatory treatments for cardiovascular conditions.
These treatments should only be used in specific situations, since they can increase the risk of infectious disease.
The authors write that “the detection of acquired mutations linked to clonal hematopoiesis could help identify those people who could benefit most from anti-inflammatory treatment, when presenting with exacerbated inflammation, and inform which specific treatment could be especially effective”.
Achieving this goal will, the authors conclude, require detailed research into the effects of different mutations associated with clonal hematopoiesis and, eventually, clinical trials of candidate treatments.
Drs. Fuster and Tall are members of the Clonal Hematopoiesis and Atherosclerosis Network, a Leducq Foundation-funded association of pioneering researchers in the field that also includes CNIC scientist Andrés Hidalgo.
Circulation
A CNIC study highlights the risks of mitochondrial therapeutic interventions
Research carried out at the CNIC has demonstrated that mixing mitochondrial DNAs (mtDNAs) of different origins can have damaging effects over the medium and long term. mtDNA is a component of the genetic material that is transmitted exclusively from mothers to their children.
The study, published in Circulation, provides invaluable information about how to identify and avoid possible risks associated with mitochondrial therapeutic interventions. The most popular of these methods include the injection of mitochondria from a donor egg into the egg of a woman with fertility problems and mitochondrial replacement therapy aimed at preventing the transmission of disease-causing mutations to descendents, popularly known as “three-parent children.” Mitochondrial replacement therapy has already been approved in the United Kingdom.
The new study shows that, while most cells do not tolerate the presence of two mitochondrial genetic variants and progressively eliminate one of the two mtDNAs, some major organs are unable to do this, including the heart, lungs, and skeletal muscle.
For lead researcher Dr. José Antonio Enríquez, who heads the Functional Genetics of the Oxidative Phosphorylation System (GENOXPHOS) group at the CNIC, the findings have major implications for treatments involving the transfer of donor mitochondria because they show that “animals generated through these procedures appear healthy early in life but go on to suffer in later life from heart failure, pulmonary hypertension, loss of muscle mass, frailty, and premature death.”
In the body, most of the DNA is contained in the cell nuclei. In humans, this is where approximately 20,000 genes of the genome are located. However, another 37 genes are located outside the nucleus. “These genes are located in cellular compartments called mitochondria and constitute the mitochondrial DNA,” explained Dr. Enríquez.
In contrast, mtDNA is inherited only from the mother because the sperm mitochondria are destroyed in the interior of the fertilized egg. Uniparental transmission of mtDNA is found in almost all organisms. In addition, mtDNA is present in multiple copies per cell, and these copies are all essentially identical, a phenomenon known as homoplasmy.
The presence of more than one mtDNA genetic variant in the cell is called heteroplasmy. Although very rare, heteroplasmy sometimes occurs naturally as a result of mtDNA mutations and can cause several diseases. New therapeutic approaches proposed in recent years and aimed at preventing disease or treating infertility can generate a new form of heteroplasmy in people.
“This new form of heteroplasmy, involving distinct non-mutated mtDNA variants, is produced when an individual’s cells contain both the original recipient mtDNA and the donor mtDNA transferred during the intervention. In the GENOXPHOS group at the CNIC, we have been investigating whether this breaching of a natural biological barrier has detectable physiological effects,” said Dr. Enríquez.
The researchers show that the selection between mtDNA variants coexisting in the same cell depends on their impact on cell metabolism and can be modulated by variations in gene function, drug actions, and dietary changes. “All of these factors help to determine the preference for one type of mitochondrial genome over another,” they write.
“The question as to why mtDNA is transmitted to descendents from only one parent has yet to be answered, but until now the issue had no health implications,” said first author Dr. Ana Victoria Lechuga-Vieco. “The new medical therapies that breach this biological barrier can generate, intentionally or non-intentionally, mixtures of mtDNA from more than one individual in the same cell”.
The study provides invaluable information for ensuring the safety of mitochondrial replacement therapies aimed at preventing the transmission of disease-causing mutations to descendents, popularly known as “three-parent children.”
Before the publication of the new study, “we did not know what impact this mtDNA mixing had for the individual,” said Dr. Enríquez.
To address this question, the GENOXPHOS group generated mice with a single nuclear genome but with all their cells simultaneously containing two distinct mtDNA variants. “This mouse strain was fertile, and young animals showed no related disease,” explained Dr. Lechuga-Vieco.
“We observed that cells rejected the presence of two mitochondrial genomes, and most of them progressively eliminated one of the mtDNA variants. Surprisingly, however, major organs like the heart, lungs, and skeletal muscle were unable to do this,” explained Dr. Lechuga-Vieco.
“Organs that could eliminate one of the mtDNA variants, like the liver, recovered their mitochondrial metabolism and cellular health, but those that could not progressively deteriorated as the animals aged,” continued Dr. Enríquez.
Thus, the animals, which appeared healthy in their youth, in later life suffered from heart failure, pulmonary hypertension, loss of muscle mass, frailty, and premature death.
The researchers conclude that the dangerous effects of mitochondrial therapeutic interventions identified in the new study show the need for caution in the selection of the donor mtDNA genotype.
As the authors state in their article, the results of the Circulation study also imply that “even the most promising method, for the replacement of oocyte mitochondria carrying known pathological mtDNA mutations, may fail to achieve 100% replacement.”
Any therapeutic strategy that involves mixing the healthy mtDNA of two individuals must ensure the genetic compatibility of the donor and recipient mitochondrial genomes.
The study shows that recipient cells have a high capacity to select and amplify the original, pre-existing mtDNA variant, which may have been undetectable before transfer of the donor mtDNA. The procedure thus has the potential to result in a mix of mtDNA from two individuals in descendent cells. “The same problem arises with oocyte rejuvenation by microinjection of donor cytoplasm,” pointed out Dr. Enríquez.
Dr. Enríquez stressed that these risks do not mean that mitochondrial replacement therapy should be abandoned. In the same way as blood transfusions and organ transplants require careful control of compatibility between recipient and donor, Dr. Enríquez recommends that any therapeutic strategy that risks the mixing of healthy mtDNA variants from two individuals should “ensure full compatibility between the donor and recipient mitochondrial genomes.”
The study was supported by the following funding bodies: CIBER de Enfermedades Respiratorias (CIBERES) and CIBER de Enfermedades Cardiovasculares (CIBERCV); Ministerio de Economía, Industria y Competitividad (MINECO); Ministerio de Asuntos Económicos y Transformación Digital (MEIC); Human Frontier Science Program; European Molecular Biology Organization; Programa Red Guipuzcoana de Ciencia, Tecnología e Información del Gobierno Vasco; and the ELKARTEK Program Department of Industry, Innovation, Commerce, and Tourism.
Nature Communications
CNIC scientists identify a shuttle protein required for the nuclear import of proteins essential for organ growth and development

Organ growth and regeneration require the entry into the cell nucleus of proteins that activate essential genes for these processes. This process is the subject of a new study by CNIC scientists, led by Dr. Miguel Ángel del Pozo Barriuso, who heads the Mechanoadaptation and Caveolae Biology group, and group member Dr. Asier Echarri Aguirre. The scientists have identified the mechanism that controls the nuclear import of these proteins in response to mechanical stimuli, such as the hemodynamic forces generated by arterial blood flow, tumor rigidity, or locomotory movements during routine activities like walking or sports. The results are published today in Nature Communications.
Most biological processes require the nuclear entry of key regulatory factors. Processes such as fetal development, tissue regeneration after trauma or infarction, cardiovascular disease, and cancer generate mechanical signals that stimulate cell multiplication to regenerate the damaged tissue or remodel the surrounding tissue matrix.
These events require specific factors that are activated by mechanical signals and enter the nucleus, where they activate the expression of genes required to promote organ growth or regeneration.
“One of the most important of these factors is the protein YAP,” explained Dr. Del Pozo Barriuso. “Its nuclear import is a highly regulated process, and only takes place when specifically required. Macromolecules like YAP enter the nucleus through nuclear pores by binding to a nuclear transport or shuttle protein.”
What makes YAP especially interesting is that, in response to mechanical forces acting on the tissue, “it is activated and enters the nucleus, where it switches on several genes that determine the growth of the affected organ,” explained Dr. Echarri Aguirre. “Moreover, YAP is mutated in many diseases, making it even more interesting,” added study first author María García.
Nevertheless, although YAP has been studied extensively due to its roles in organ regeneration, cardiovascular disease, and cancer, its nuclear entry route and the nuclear shuttle protein it interacts with were unknown.
The CNIC scientists have now shown that the YAP shuttle protein partner is importin-7, which binds YAP and transports it to the nucleus, where it can induce cell and tissue growth.
The study have also demonstrated that YAP monopolizes importin-7, thereby restricting nuclear access by other factors. The study thus shows that YAP not only controls genes important for organ growth, but also regulates nuclear shuttle activity and the nuclear import of other factors.
The study thus identifies a new target for the development of drugs targeting YAP nuclear import in diseases associated with an enormous societal and economic cost.
Scientists of the CIBER Cardiovascular Disease research network participated in this study. The study was supported by funding from the following bodies: Ministerio de Ciencia, Innovación y Universidades; Agencia Estatal de Investigación / European Regional Development Fund “A way to make Europe”; the Comunidad Autónoma de Madrid; Fundació La Marató de TV3; Fundación “la Caixa”; Asociación Española Contra el Cáncer; and the European Union’s Horizon 2020 Research and Innovation Programme through a Marie Sklodowska-Curie award.
EHJ
Bone marrow contributes to the development of atherosclerosis
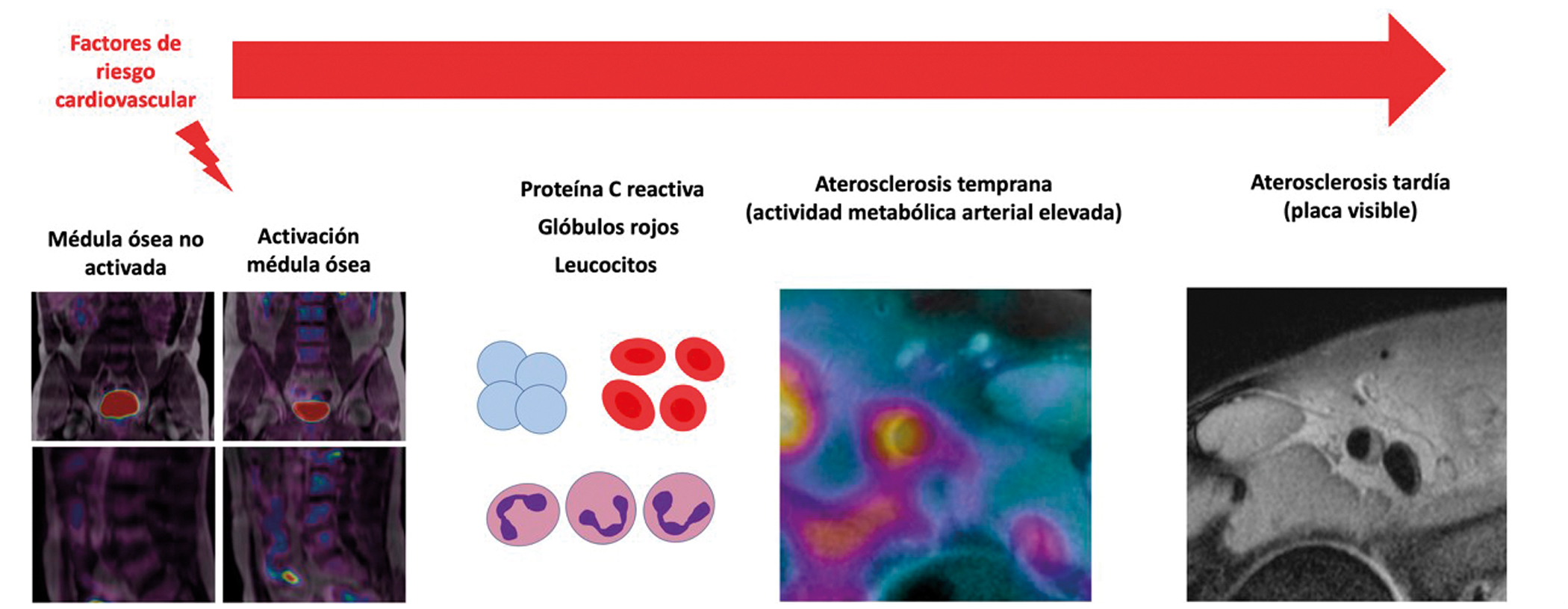
The activation of the bone marrow appears to play a key role in the origin and development of atherosclerosis, the pathological process underlying cardiovascular conditions such as myocardial infarction and stroke. A study carried out by scientists at the CNIC and led by cardiologists Valentín Fuster and Borja Ibáñez suggests that the bone marrow is activated in response to known cardiovascular risk factors. In the study, published in the European Heart Journal, the researchers show that these risk factors lead to an increase in the number of circulating inflammatory cells, which go on to trigger the initiation and subsequent progression of atherosclerotic disease.
Atherosclerosis is a progressive deposition of fats and inflammatory cells in the arterial wall, resulting in the formation of atheroma plaques. Is the most frequent cause of death in the world and is considered a ‘silent killer’ because it develops quietly for years before producing symptoms. Identifying atherosclerosis in its initial phases, before symptoms appear, is a major goal of the PESA-CNIC-Santander (Progression of Early Subclinical Atherosclerosis) study. This study was launched in 2010 as a collaboration between the CNIC and Santander Bank and is led by Dr. Valentín Fuster, CNIC Director General and cardiologist and Medical Director at Mount Sinai Hospital in New York.
Cardiovascular risk factors that activate the bone marrow are those linked to metabolic syndrome: central obesity (the accumulation of abdominal fat), a high blood triglycerides, low HDL cholesterol, elevated blood glucose, insulin resistance, and high blood pressure.
These factors trigger an increase in metabolic activity in the blood marrow that can be detected with the advanced image techniques available at the CNIC, such as hybrid positron emission tomography (PET)–magnetic resonance imaging (MRI). “The increased metabolic activity of the bone marrow unleashes an inflammatory process that activates atherosclerosis, from its earliest stages through to the appearance of established plaques,” said Borja Ibáñez, CNIC clinical research director, cardiologist at Hospital Universitario Fundación Jiménez Díaz, and a group leader in the Spanish cardiovascular research network (CIBERCV), who directed the study together with Fuster.
PESA-CNIC-Santander is considered one of the most important cardiovascular prevention studies in the world. As Dr. Valentín Fuster explained, “PESA is the CNIC’s flagship study, providing a focus for many of the center’s pioneering research groups, each a leader in a defined area of cardiovascular disease. This joining together of basic and clinical researchers to investigate a large cohort like PESA is a unique venture in cardiovascular research.
eBiomedicine
Scientists discover a new method for the early detection of subclinical atherosclerosis

A study published in the journal eBiomedicine identifies new biomarkers that predict the presence of subclinical atherosclerosis. The study was carried out by scientists from the Spanish cardiovascular research network (CIBERCV ) working at the CNIC and the Instituto de Investigación Sanitaria-Fundación Jiménez Díaz-Universidad Autónoma de Madrid (IIS-FJD-UAM), in partnership with other institutions.
“Atherosclerosis is a leading cause of cardiovascular disease, which is one of the major health problems in the world and places an enormous burden on health care systems. It is therefore a major goal to identify the disease in its earliest phases, so that interventions can halt its progression before it reaches an advanced stage,” said study coordinator Jesús Vázquez, lead investigator of the CIBERCV and head of the Cardiovascular Proteomics laboratory at the CNIC.
Early prevention is the best approach to combatting the cardiovascular disease pandemic. Atherosclerosis has a long preclinical phase and is usually diagnosed only at advanced stages, often after a cardiovascular event. The use of noninvasive imaging techniques to detect atherosclerosis allows a more precise stratification of risk than is possible with conventional methods, and current clinical guidelines recommend the use of imaging techniques to assess individual risk in combination with scales based on traditional risk factors, especially in individuals at low-to-moderate risk according to these scales.
Nevertheless, cardiovascular imaging techniques are not universally available, and the extent of atherosclerosis varies substantially between individuals in the same traditional risk category. There is therefore much interest in developing alternative rapid and noninvasive methods to estimate atherosclerosis burden.
Plasma biomarkers that track subclinical atherosclerosis, like the ones described in the eBiomedicine study, provide a way to sidestep the limitations of imaging approaches and to improve the prediction of cardiovascular risk
In the new study, the research team analyzed a collection of 880 blood plasma samples obtained from PESA CNIC-Santander study participants. The samples were examined by proteomic techniques with the aim of identifying circulating biomarkers of atherosclerosis in its early, asymptomatic phase.
From an initial panel of candidate biomarkers detected in this analysis, the team selected three proteins for validation in a collection of more than 3,000 plasma samples from the ILERVAS cohort. These samples were screened using rapid and widely available techniques through a partnership with The Binding Site and Hospital Quirónsalud Madrid.
Summarizing the findings, José Luis Martín Ventura, a CIBERCV scientist at the IIS-FJD-UAM and one of the study coordinators, said “the main contribution of this study is the development of a biomarker panel that can identify the presence of atherosclerosis in healthy, asymptomatic people, including individuals with none of the classical cardiovascular risk factors.”
“The CNIC group has broad experience is the use of proteomic techniques for the massive analysis of samples from patients with different cardiovascular conditions, and we have worked for several years with our colleagues at IIS-FJD on the identification and validation of cardiovascular biomarkers,” explained Jesús Vázquez.
“Imaging techniques allow the efficient detection of atherosclerosis, but these methods are costly and require highly trained personnel and specialized apparatus, which are not available in some regions and countries,” said Vázquez.
This is the largest study to date to explore the association between plasma protein concentrations and subclinical atherosclerosis using high-performance unbiased quantitative proteomics. The study demonstrates the potential of proteomics linked to mass-spectrometry for the discovery of human disease biomarkers.
The study received funding from the Ministerio de Ciencia, Innovación y Universidades through the Instituto Carlos III de Salud-Fondo de Investigación Sanitaria, CIBERCV y CIBERDEM, Fundació La Marató de TV3 and Fundación “la Caixa”.
The PESA study is co-funded equally by the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Banco Santander. The ILERVAS study was funded by Lleida provincial council.
JACC
3D matrix ultrasound accurately identifies cardiovascular injury in healthy individuals
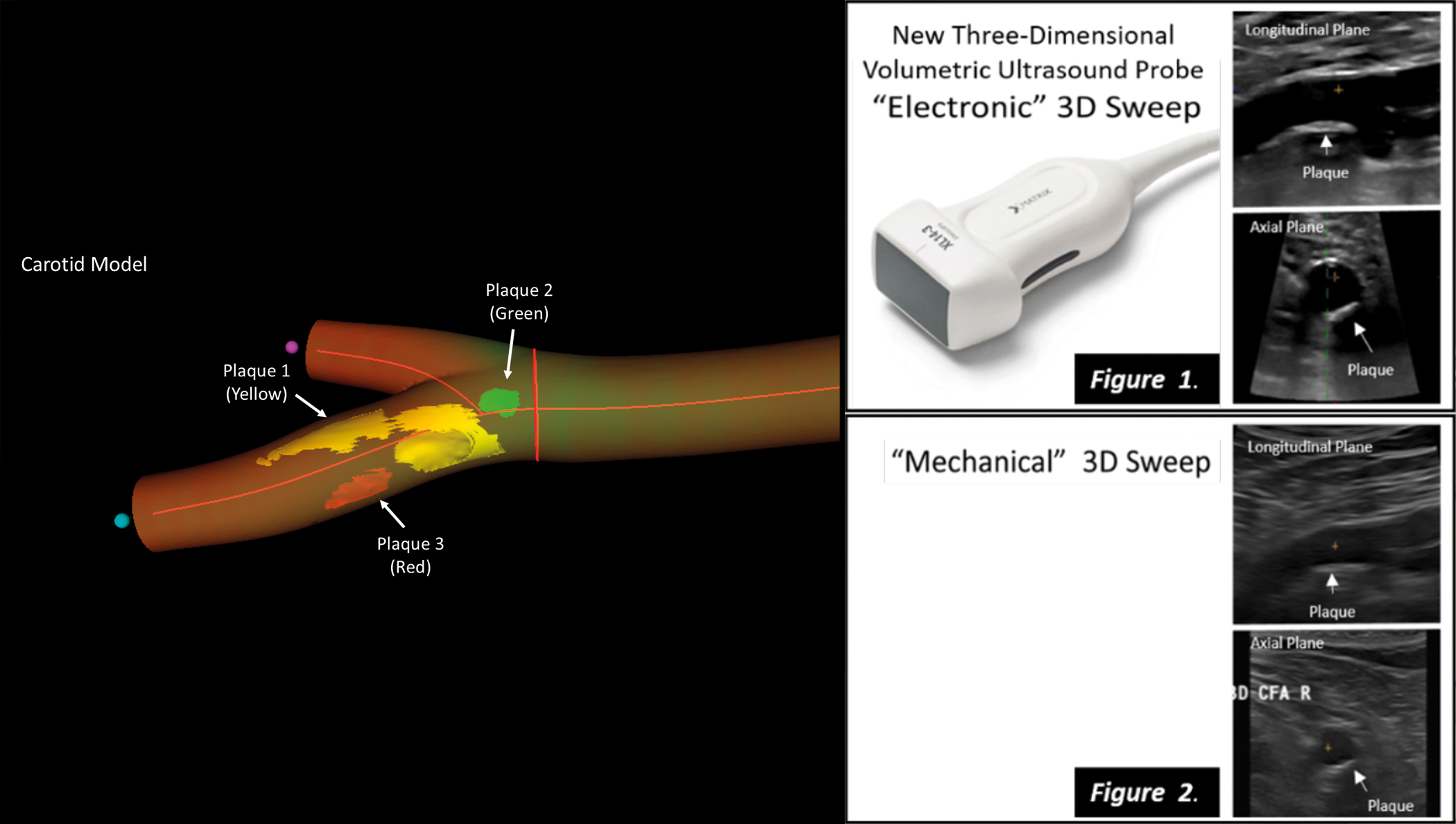
A new imaging technique for real 3D vascular ultrasound could become a key tool in strategies aimed at preventing cardiovascular disease in apparently healthy persons, complementing traditional risk parameters such as cholesterol and high blood pressure. The new results, published in JACC: Cardiovascular Imaging, show that real 3D vascular ultrasound is reliable, accurate, and faster than previous methods for the assessment of plaque volume in the carotid and femoral arteries.
The new imaging method was first validated and implemented in a study of almost 200 healthy participants with an intermediate cardiovascular risk from the Athero Brain: Head-to-Heart study, led by Dr. Valentín Fuster, Director General of the Centro Nacional de Investigaciones Cardiovasculares (CNIC). The method has now been incorporated into the PESA-CNIC-Santander study, also led by Dr. Fuster, where it is being used to assess more than 4,000 healthy individuals over a 9-year follow-up.
The CNIC researchers partnered with Philips Ultrasound and Philips research Paris-Medisys to develop a new probe and software for real 3D ultrasound to facilitate exploration of the carotid and femoral arteries and speed up quantification of atherosclerotic plaque volume. As Dr. Fuster explained, “it is clear that traditional clinical evaluations based on measurements of cholesterol, blood pressure, blood glucose, and lifestyle habits cannot, on their own, accurately determine accumulated damage in the cardiovascular system, and without this crucial information we cannot take appropriate decisions to prevent acute events such as myocardial infarction or stroke.”
The newly validated 3D vascular probe incorporates 3D matrix technology, which underpins the most advanced 3D ultrasound techniques. CNIC clinical research director Dr. Borja Ibáñez explained that the new technology allows simultaneous analysis by 2D and 3D ultrasound, includes all functionalities (color doppler, power-doppler, and contrast ultrasound), and is easily incorporated into daily clinical practice by technical and medical teams already experienced in ultrasound, emphasizing that “the integrated analysis software incorporates real 3D data processing.”
In addition to demonstrating the accuracy of 3D matrix ultrasound, the study demonstrates that the new technique takes just half the time needed by previous methods to obtain all the information required for the definition of carotid and femoral plaque burden, essential information for correct patient management.
For patients, the outstanding feature of the new method is that the software generates a virtual 3D image of their own arteries, allowing them to see the accumulated damage. “When patients see the state of their arteries, this impresses upon them the need to change their lifestyle, in graphic manner not achieved by reading a list of analytical data,” said first author Dr. Beatriz López Melgar, a cardiologist at Hospital Universitario La Princesa and head of the 3D Cardioprevention Program at Hospital HM Montepríncipe in Madrid.
López Melgar concluded that, with the development of this technology, “we now have a tool that can be used on-the-fly in an initial consultation, speeding up decision making—an important consideration in cardiovascular prevention, where time is of the essence.”
Collaborators on this project included scientists from the Spanish cardiovascular research network (CIBERCV), and financial support was provided by the Ministerio de Economía, Industria y Competitividad (MEIC) and the European Regional Development Fund.
NATURE
International team led by BGI completes first whole-body cell atlas of a non-human primate
In a breakthrough that could lead to scientific advancement in the treatment of human diseases, researchers from BGI-Research, Jilin University and the Guangzhou Institutes of Biomedicine and Health (Chinese Academy of Sciences), together with scientific research teams from 35 international institutions including China, Germany, Italy, Singapore, Spain, Sweden and the UK, published the world’s first non-human primate whole-body cell transcriptomic atlas in the scientific journal Nature. The study, Cell transcriptomic atlas of the non-human primate Macaca fascicularis, obtained ethical clearance before it was conducted.
By using BGI’s independently-developed DNBelab C4 single-cell library sequencing platform, the researchers completed the single-cell transcriptome of 45 tissues and organs from long-tailed macaque (cynomolgus) monkeys, obtaining a total of 1.14 million single-cell data and identifying 113 major cell types.
Non-human primates such as macaques are the species closest to humans in the evolutionary tree. By mapping the macaque transcriptome at the single-cell level, scientists now have a database, or single-cell library, that can be used for developing methods for disease diagnosis and treatment, assessment of clinical drug efficacy, analysis of cell evolution among species, and analysis of advanced cognitive functions of the brain.
Single-cell mapping allowed the team to identify the cell types that may contribute to human disease or make individuals more susceptible to the disease. For example, in COVID-19, the biggest manifestation is pneumonia because SARS-CoV-2 infects a small group of cells in the lung. However, the single-cell mapping of macaque also identified certain cells in other tissues that can become infected in primates. This can help doctors understand where to look for signs of COVID-19.
Single-cell mapping can also help identify which cells metabolize calories from fat, allowing researchers to comprehend underlying contributors to obesity. Likewise, this process could help identify which cells regulate neuronal circuits in the brain, leading to potential treatments for neurological diseases.
“Single-cell research is transforming our understanding of tissue and organ functions at a cellular level, which informs how diseases develop and how they can be treated” and “having a whole-body organ single-cell map of the adult macaque will significantly improve the ability to pinpoint how to develop potential treatments for human diseases with greater precision” said Pura Muñoz-Cánoves, one of the corresponding authors of the paper.
“By understanding cell types and their characteristics, scientists will be able to predict the impact of disease treatments on specific cell structures and thus develop more targeted approaches for monogenic or complex genetic diseases,” said co-corresponding author Dr. Xu Xun, director of BGI-Research.
“This study fills the gap of the single-cell map of non-human primates and is a rich data resource that will serve as a very important reference for future species evolution, brain science, drug evaluation and screening, and preclinical research studies” said another co-corresponding author, Dr. Miguel A. Esteban from Jilin University and the Guangzhou Institutes of Biomedicine and Health (Chinese Academy of Science).
REDOX BIOLOGY
Discover a new mechanism involved in the modulation of heart muscle elasticity

Scientists at the CNIC, in collaboration with an international scientific team, have described a new mechanism of modulation of the mechanical properties of the heart, based on the oxidation of the protein titin, which is the main protein responsible for the passive elasticity of the heart muscle.
Titin is the largest protein in the human body and it is a key protein for the function of skeletal muscle and the heart. “Simplifying a lot, we can describe titin as a molecular spring that allows muscle cells to stretch and contract,” explained Dr. Jorge Alegre Cebollada, who leads the Molecular Mechanics of the Cardiovascular System laboratory at the CNIC.
The study, published in Redox Biology, builds on earlier observations showing that oxidation of the amino acid cysteine modulates the mechanical properties of titin in vitro. “We wondered whether these oxidations might be present in vivo and help to explain how the heart adapts mechanically to different situations and how it responds to disorders that alter the oxidative balance,” explained Dr. Alegre Cebollada.
“We first found that titin contains a set of cysteines that are highly evolutionary conserved, suggesting that they play an important role in the function of the protein,” commented Dr. Elías Herrero Galán, codirector of the study. This set of conserved cysteines are the ones observed to modulate the mechanical properties of titin in vitro. “Our experiments also showed that these amino acids are a target for oxidation in basal physiological conditions both in the mouse and the human heart”, said Dr. Herrero Galán. This mechanism provides a possible explanation for alterations affecting the heart’s oxidative state, such as myocardial infarction
Doctoral Student Inés Martínez Martín described how they identified the effects of these oxidations by running computer simulations based on mathematical models: “Depending on the type of oxidation, titin becomes more or less stiff, affecting the mechanics of the myocardium”. “In general,” she added, “these oxidations make titin more dynamic and malleable, allowing the heart to adapt to different metabolic and oxidative demands”.
The authors propose that this mechanism might also explain the alterations that occur in the heart during pathological processes that affect its oxidative state, such as myocardial infarction.
The study was supported by funding from the Spanish Ministerio de Ciencia e Innovación, Madrid Regional Government, and Fundación “la Caixa”. The study also received funding from the European Research Area Network on Cardiovascular Diseases through the MINOTAUR project.
Nature Cardiovascular Research
A CNIC team creates a dynamic 3D atlas of the formation of the embryonic heart
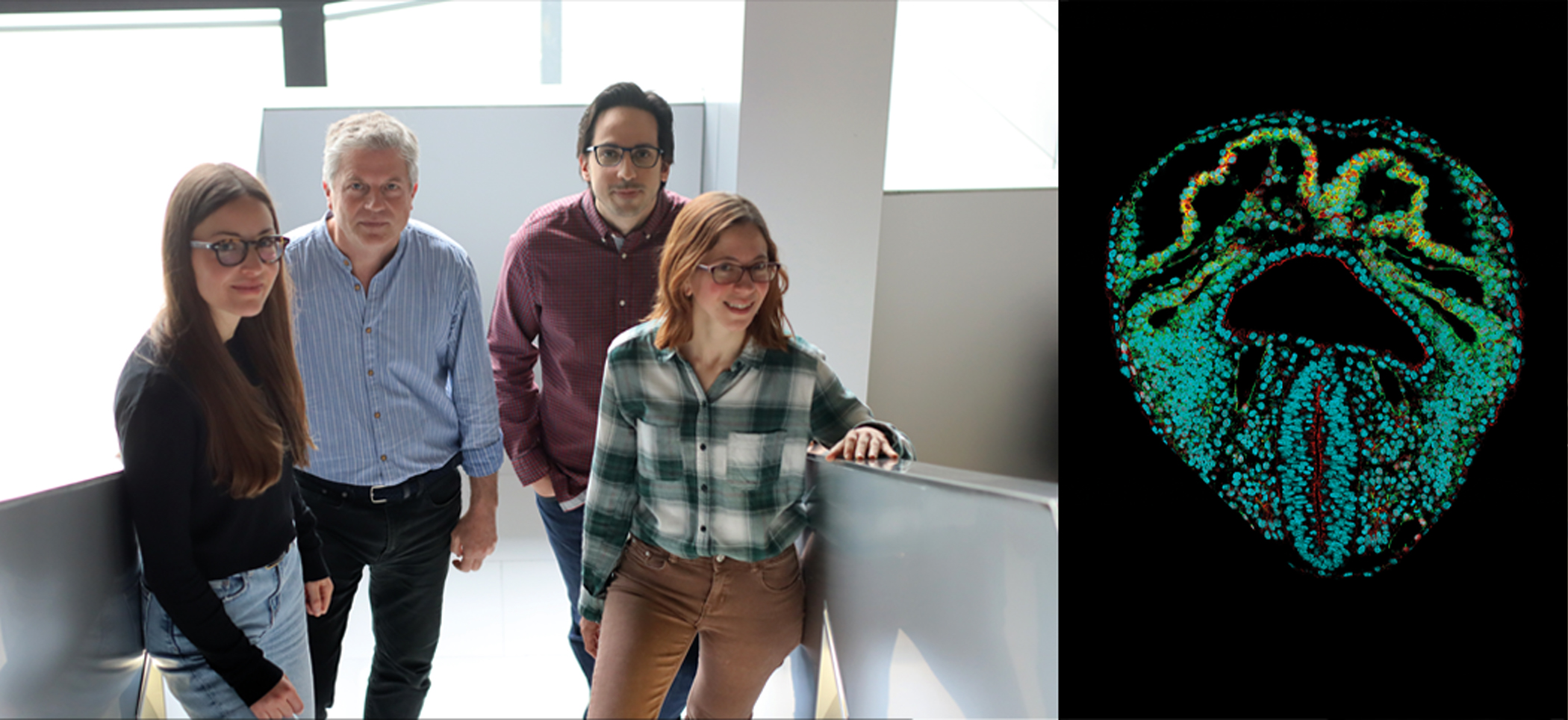
Scientists at the CNIC have used a collection of mouse tissue samples to create a 3D atlas of the formation of the heart during embryonic and fetal development. The 3D atlas has allowed the scientists to identify the first appearance of left–right asymmetry in the heart. The study, published in Nature Cardiovascular Research, provides important information on the development of congenital heart malformations.
Study leader Dr. Miguel Torres, whose lab specializes in the Genetic Control of Organ Development and Regeneration, said that the new study “will be of immense help to researchers seeking to understand heart development.”
During the early phases of cardiogenesis, no two embryonic hearts are alike, and the differences can be so great that it can be difficult to decide which is at a more advanced stage of development. Nevertheless, assured Dr. Torres, “the early, apparently divergent morphologies later converge to produce the well-formed, typical heart of the newborn animal.”
The challenge is to gauge the average progression of cardiac geometry from the wide variability encountered in nature and to learn to discriminate between the various physiological morphologies and anomalous forms. Doing this requires access to a sufficiently large sample collection.
To get around the difficulties of obtaining live images, the CNIC team acquired a large collection of high-resolution images of embryonic mouse hearts at key developmental stages and at a high temporal density.
“We realized that we could not examine the morphogenesis of the heart in isolation from that of the adjoining tissues, because the formation of the heart tube can be likened to a geological fold, produced in a continuous layer of mesoderm (one of the cell layers in the early embryo) in the pericardiac cavity,” said Dr. Torres. First author IsaacEsteban described how the team therefore “captured images of all the tissues in the pericardiac cavity, as well as the endoderm of the underlying anterior intestine.”
The investigators converted all the images into digital form and, using a morphometric staging system, ordered them into a temporal series, a superior approach to relying on the moment at which embryos were obtained, which does not necessarily correspond to the true timing of morphological development.
The CNIC scientists used a recently developed approach called mapping between surfaces. This technique generates corresponding surface point maps of similar structures at a high point density that allows reconstruction of the entire surface of the objects.
“This method allowed us to identify equivalent positions in groups of specimens from a similar developmental stage or at consecutive developmental stages,” said Esteban. Using this approach, the team created a temporal 3D atlas that shows the trajectory of the formation of the heart tube and reveals local morphological variability at each stage.
The atlas reveals that the regions usually involved in heart malformations are also the regions that show high morphological heterogeneity or variability during development. “This observation suggests that this morphological variability could underlie the high incidence of congenital heart malformations, which affect 1% of live newborn babies,” said Esteban.
The same approach used in this study can also be used to measure the morphogenesis of any organ or organoid (an in vitro model of a developing organ).
Dr Torres remarked that, while a picture may be worth a thousand words, “a movie provides much more information than a thousand individual images,” and affirmed that “the dynamic 3D atlas and the morphometric staging system described in the study will be immensely useful and a source of inspiration for scientists interested in understanding the development of the heart.”
The main limitation of the study is that it reconstructs a dynamic process from fixed images, and so does not reveal details of the cellular basis of tissue deformation. Nevertheless, the atlas will be indispensable for efforts to define these cellular interactions, and scientists are already working to incorporate cell data into the new dynamic atlas of heart development.
eLife
Study reveals how Duchenne muscular dystrophy causes heart rhythm problems
Abnormalities in the proteins responsible for transmitting electrical signals in the heart likely cause abnormal heart rhythms in patients with Duchenne muscular dystrophy (DMD), shows a study published in eLife.
The results help explain why as many as 60% of patients with DMD have potentially life-threatening heart rhythm abnormalities. They may also suggest potential treatment strategies for heart problems in people with DMD.
Mutations in a gene that encodes a muscle-protecting protein called dystrophin cause DMD. The condition disproportionately affects males who inherit one copy of the abnormal gene from their mothers. Without functioning dystrophin proteins, patients develop progressive muscle loss. Women who often inherit one functional and one dysfunctional gene copy may have less severe muscle deterioration. But both men and women with DMD are at high risk of life-threatening heart rhythm abnormalities.
“No one knows why patients with DMD develop heart rhythm abnormalities,” explained lead author Eric Jiménez-Vázquez, an assistant research scientist at the Center for Arrhythmia Research at the University of Michigan in Ann Arbor, Michigan USA. “We set out to determine the role of ion channels, which control electrical signals in the heart.”
The study was a collaboration of physicians and scientists from four different laboratories in three different countries (the University of Michigan in the USA, the Centro Nacional de Enfermedades Cardiovasculares (CNIC) in Spain, and the Sheba Medical Center and the Technion Institute in Israel).
They collected skin biopsies of three people with DMD and two healthy volunteers without DMD. Two participants with DMD were males who inherited an abnormal copy of the gene for dystrophin, and one was a woman with one mutant copy and one normal copy of the gene. To determine why the patients developed arrhythmias, in the laboratory, they converted the biopsied cells into stem cells and then coaxed them to become heart muscle cells.
When they measured electrical activity in the newly formed heart cells, they found that cells from people with DMD had slower electrical signals, generated arrhythmias and were less able to contract than cells from people without the condition. Individuals with DMD also had less potassium and sodium ions flowing in their cell membranes, both essential for electrical signalling in the heart. They also found that heart muscle cells grown from the males with DMD had fewer sodium and potassium ion channels, which control the flow of sodium and potassium, than people without the condition.
But adding an essential partner protein of sodium and potassium channels called α1-syntrophin to the cells of one of the males with DMD corrected electrical activity in the cells and prevented abnormal rhythms.
The discoveries may help explain why both males and females with DMD may have life-threatening heart rhythm disturbances. In DMD, males are more often affected but females may be carriers because the dystrophin gene is located on the X chromosome. Since males have one X and one Y chromosome, if they inherit the DMD mutation from their mother’s X chromosome, they do not have a way to make any active dystrophin protein. Thus, they have a high probability of developing the disease. Since females have two X chromosomes, they do not usually have disease symptoms because they can still make dystrophin from their good X chromosome. However, the pattern of distribution of the bad chromosome in the heart cells is random and may determine the severity of their symptoms. This inconsistent pattern may also explain why women with DMD are sometimes as prone to heart rhythms as their male counterparts.
“Our study helps explain why people with DMD develop severe heart rhythm abnormalities and may help scientists develop new treatments for this life-threatening complication,” said senior author José Jalife, distinguished senior investigator at the CNIC and emeritus professor of Medicine and Molecular and Integrative Physiology at the University of Michigan.
The study was supported by National Institutes of Health; Fundación “la Caixa”; Fundación La Marató de TV3: Ayudas a la investigación en enfermedades raras 2020 (La Marató-2020); Instituto de Salud Carlos III/FEDER/FSE- Horizonte 2020 - Programa Marco de Investigación e Innovación - Research and Innovation Framework Programme; and Ministerio de Ciencia e Innovación (MCIN).
Hepatology
Immune cells in the liver regulate body temperature
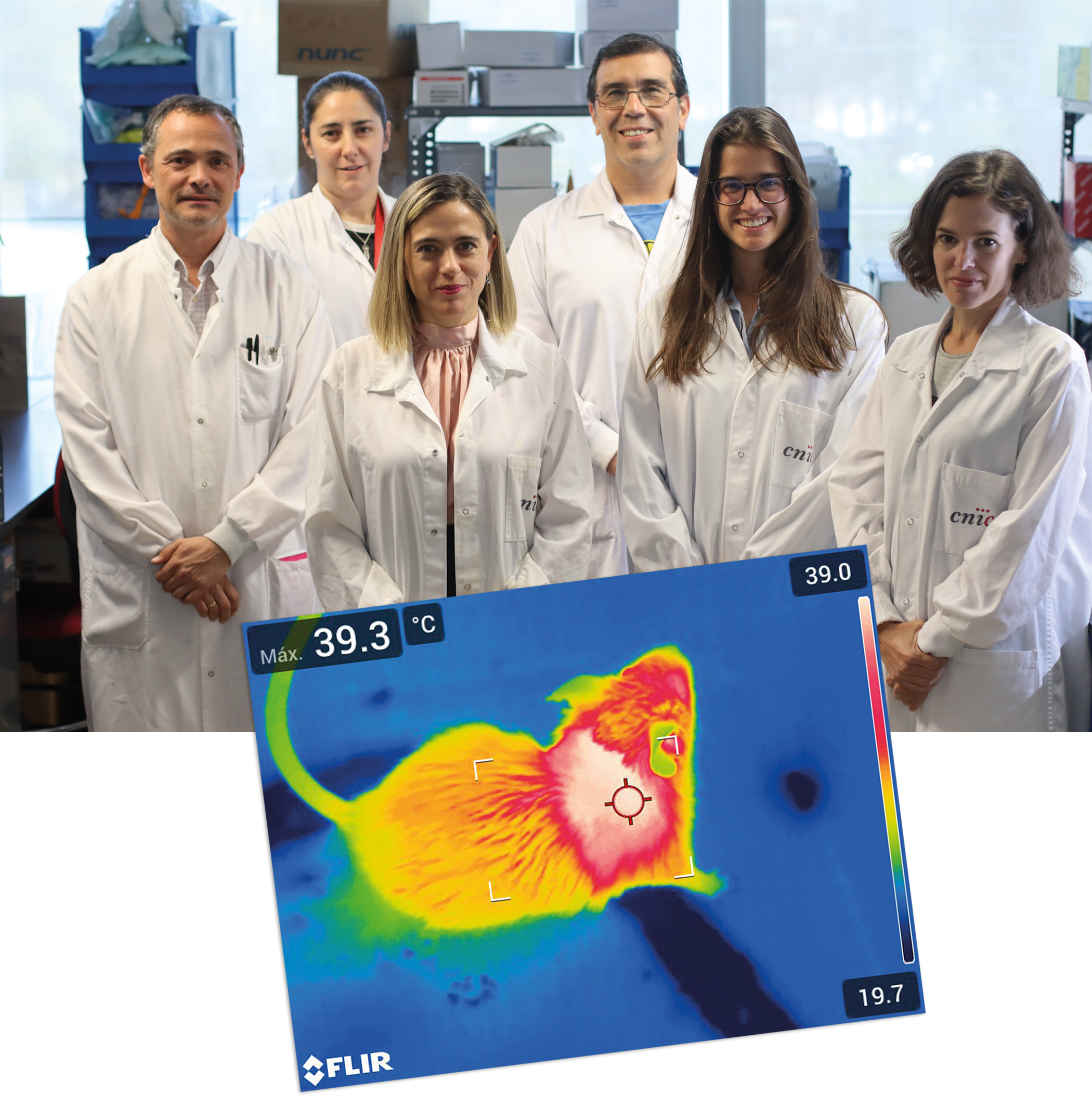
A study published in Hepatology demonstrates that the activation of thermogenesis in the livers of obese mice contributes to weight loss and improves diabetes symptoms.Scientists at the CNIC have discovered a complex network of connections between tissues that allows the liver to regulate body temperature. The research team found that the secretion of a molecule called interleukin 12 (IL-12) by immune cells present in the liver reduces heat generation by brown fat.
The new study reveals that one of these molecules, the protein IL-12, blocks the production of another protein, FGF21, in the liver. The decline in FGF21 results in reduced generation of heat by brown fat. The Spanish scientists also discovered that the production of IL-12 by these immune cells is activated by the protein kinase p38, thus identifying a role for p38 in the regulation of liver endocrine function and whole-body metabolism. “We found that when we eliminated stress-kinase pathways in macrophages, these cells lost the capacity to adapt to a fatty diet and increased the production of IL-12, reducing the generation of heat,” explained María Crespo, first author on the study.
The inflammatory component of obesity is very important because it contributes to the development of diabetes, fatty liver disease, and liver cancer. The study shows that IL-12 secreted by liver-infiltrating macrophages blocks the production of FGF21, a hormone produced in the liver that regulates body energy expenditure by promoting thermogenesis in brown fat. Although other groups have reported that thermogenesis is regulated by macrophages in fat tissue, this is the first study to demonstrate the regulation of thermogenesis by macrophages in another organ, and through a completely different mechanism.
The article also describes the link between IL-12 and the prognosis of patients with fatty liver disease. As study leader Dr. Guadalupe Sabio explained, “we found that IL-12 is elevated in the livers of obese patients, and this is directly associated with worse liver damage. This result indicates that IL-12 contributes to the progression of steatosis and could be used as a new disease marker and as a target for the treatment of this disease.”
The study highlights the importance of thermogenesis in the control of obesity, since activation of this process burns energy, promoting weight loss and ameliorating diabetes. As the authors point out, there are already drugs on the market that reduce the levels of IL-12, and these could in principle be used to treat patients with obesity or metabolic syndrome.
In addition to the CNIC group led by Dr. Sabio, the study also included contributions from scientists in the Spanish research network on the pathophysiology of obesity and nutrition (CIBERobn), the Universidad Rey Juan Carlos, and Hospital Universitario de Salamanca.
The study received funding from the following grant awarding bodies: Asociación Española Contra el Cáncer, EFSD, Lilly European Diabetes Research Programme; Ministerio de Ciencia, Innovación y Universidades; Comunidad de Madrid; Fundación BBVA; European Union 7th Framework Programme.
Science Advances
The genes that give the embryonic cells the maximum potential also control the process of their differentiation
The genes that make a cell pluripotent and have the capacity to become any other type of cell during the embryonic stage also influence the process by which cells choose their cellular identity and that of their descendants. This is demonstrated by a study conducted by the CNIC, in collaboration with the Severo Ochoa Center for Molecular Biology, a joint center of the Higher Council for Scientific Research (CSIC) and the Autónoma University of Madrid (UAM), whose results have been published in Science Advances.
After fertilization, the zygote is generated, a single cell from which a complete organism will develop. In the first divisions, the cells are equivalent, they have a great capacity to proliferate and a plasticity that will allow them to develop cells as different as a neuron or a hepatocyte. However, this power is progressively lost throughout embryonic development, as the cells make lineage decisions and differentiate themselves.
Until now it was thought that these genes only made the pluripotent cell, but we have seen that they also influence the next process, that of differentiation. After implantation in the uterus, the embryo undergoes a process called gastrulation, in which the cells choose their fate within the body and at this point pluripotency as such ceases to exist. However, the expression of pluripotency factors continues a little longer in time and our goal has been to understand why these factors continue to be expressed and what their function is, beyond pluripotency,” explains the researcher, Miguel Manzanares.
The ability to generate any type of cell is maintained in the embryo thanks to the action of the so-called pluripotency factors (OCT4, NANOG and SOX2, among others) that are only expressed in the early stages of development, while in an adult organism they are turned off.
The current research has focused mainly on OCT4, which is one of the four factors that in 2006 were overexpressed by Shinya Yamanaka to reprogram adult fibroblasts (cells that contribute to tissue formation) and convert them into induced stem cells. She was awarded the Nobel Prize in Physiology and Medicine in 2012 for this research.
To study this process in detail, the research team has characterized, both in vivo and in vitro, how the OCT4 factor influences the regulation of Hox genes, which are responsible for attributing identity to the cells of the anteroposterior axis of the embryo.
“There are many projects on pluripotency, however very little is known about how this network is disassembled to allow determination of a specific cell lineage. Understanding pluripotency is the Rosetta stone for unraveling many important and complex processes such as tissue regeneration, rejuvenation or cancer”, concludes Manzanares.
EJHF
Spanish scientists combine genetic and imaging data to improve the treatment of dilated cardiomyopathy

Combining a person’s genetic profile with imaging data obtained by cardiac magnetic resonance accurately predicts the prognosis of patients with dilated cardiomyopathy, the most frequent cause of heart failure.
This is the finding of a Spanish study published in the European Journal of Heart Failure and coordinated by Dr. Pablo García-Pavía, cardiologist at the CNIC and Hospital Puerta de Hierro Majadahonda in Madrid, and a member of the Spanish research network on cardiovascular disease (CIBERCV). The study is the largest in the world to correlate clinical outcomes with the genetic profiles and cardiac magnetic resonance data of dilated cardiomyopathy patients.
Dilated cardiomyopathy is the most frequent cause of heart disease in young people and the leading cause of heart transplantation in the world. The disease affects 1 person in every 250 of the general population and is characterized by an enlargement of the heart accompanied by a decline in its capacity to pump blood. Patients with this condition are at high risk of arrhythmias and sudden cardiac death.
The study examined genetic and cardiac magnetic resonance data collected from 600 patients in 20 Spanish hospitals between 2015 and 2020. The investigators demonstrated that a combination of specific genetic traits with the presence of fibrosis detected by cardiac magnetic resonance imaging accurately identifies those patients who will develop malignant arrhythmias or severe complications of heart failure.
The new study shows that classifying patients according to their genetic profile and the presence of fibrosis detected by cardiac magnetic resonance imaging “gives a much more accurate indication of patient risk than the extent of weakening of cardiac pumping capacity, the method used until now; the problem with the current method is that some patients with low-grade cardiac weakening develop complications, whereas others with extensive weakening are stable and don’t develop problems over the long term,” said Dr. García-Pavía.
The researchers found that patients lacking genetic alterations and showing no sign of fibrosis had a very good prognosis, with little risk of sudden death irrespective of the weakening of cardiac pumping capacity.
According to the authors, the study opens the way to a more personalized approach to dilated cardiomyopathy, with each patient receiving the most appropriate treatment based on a precise cardiological assessment. “The findings of this study allow dilated cardiomyopathy patients to be treated according to their specific characteristics and open the way to the application of personalized medicine in this area of cardiology,” concluded Dr. García-Pavía.
The following centers participated in the study: Hospital Universitario Puerta de Hierro, IDIPHISA; CIBER Cardiovascular; Hospital General Universitario Gregorio Marañón; Instituto de Investigación Biomédica de Salamanca (IBSAL) - Complejo Asistencial Universitario de Salamanca; Universidad de Salamanca; Hospital Universitario Virgen de la Arrixaca de Murcia; Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona; Complejo Hospitalario de Navarra; Instituto de Investigación Biomédica de A Coruña (INIBIC), Complexo Hospitalario Universitario de A Coruña; Universidad de A Coruña; Complejo Hospitalario Universitario de Cáceres; Hospital Universitario Virgen de la Victoria IBIMA, Málaga; Instituto de Ciencias del Corazón (ICICOR); Hospital Clínico Universitario de Valladolid; Hospital General Universitario de Alicante, Instituto de Salud e Investigación Biomédica; Hospital Universitario 12 de Octubre, Instituto de Investigación i+12; Hospital Clínico, IDIBAPS, Universitat de Barcelona; Instituto de Investigación Sanitaria de Santiago; Complexo Hospitalario Universitario de Santiago; Hospital Universitario Virgen de las Nieves; Hospital Universitario Son Llàtzer & IdISBa; Hospital Universitario Virgen del Rocío; Hospital Univesitari Dr. Josep Trueta; Instituto del Corazón & Hospital Universitario Germans Trias, and Universidad Francisco de Vitoria (UFV).
The study was funded by grants from the Instituto de Salud Carlos III with cofunding from the Euroean Regional Development Fund (“A Way to Build Europe”) and the European Social Fund (“Investing in Your Future”).
European Heart Journal
“One change a day makes 365 changes in a year”
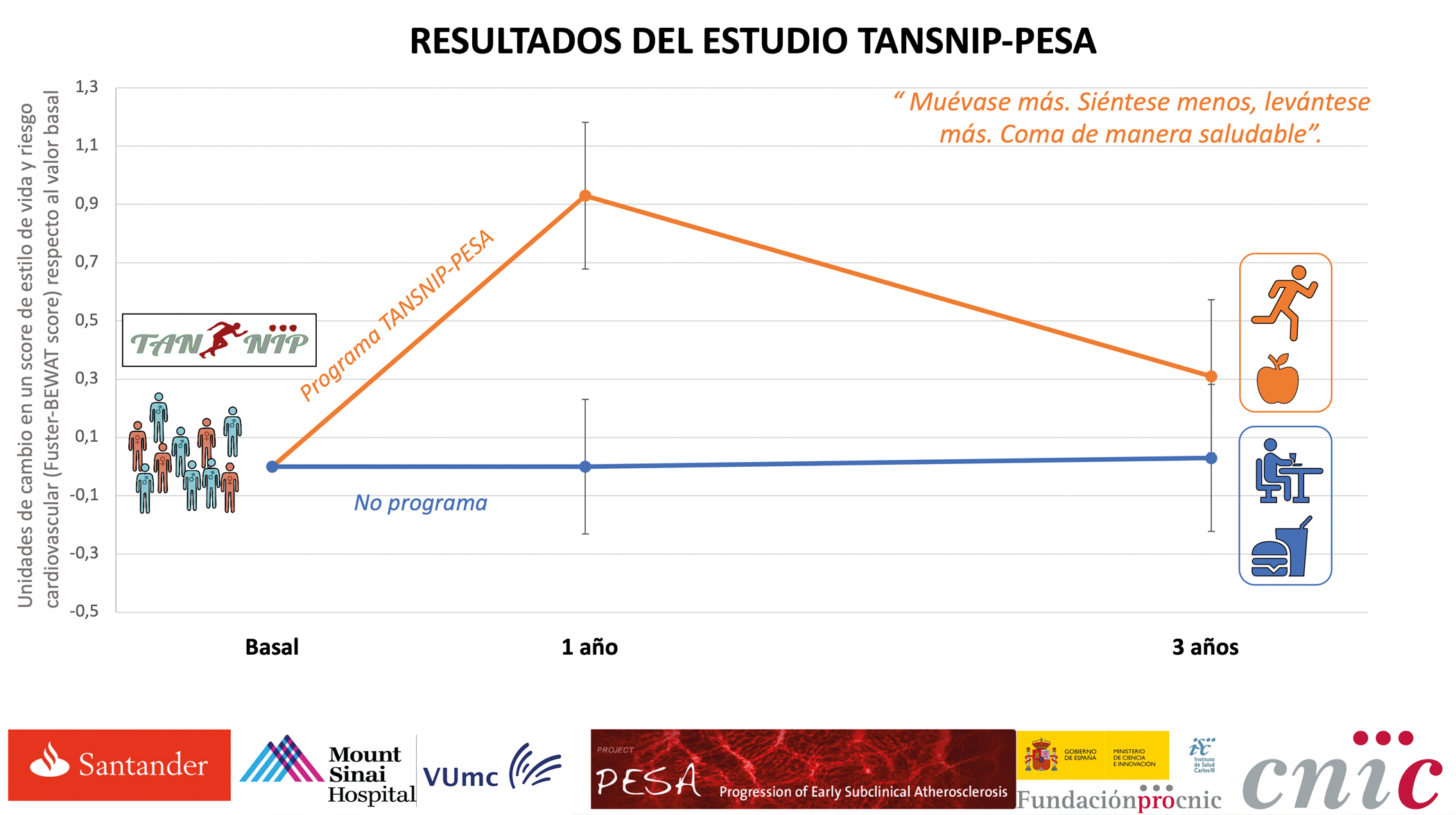
Many cardiovascular disorders can be prevented by taking action to reduce risk factors. Making even small behavioral changes and sticking with them over the long term can help to preserve cardiovascular health. This is the conclusion of a study conducted at the CNIC and published in the European Heart Journal. The study also demonstrates that the workplace is an ideal setting for programs promoting the adoption of heart-healthy habits and producing major health benefits.
A few years ago, CNIC General Director Dr. Valentín Fuster launched the TANSNIP project, an international initiative that includes partners in the United States (Icahn School of Medicine at Mount Sinai Hospital in New York and the Framingham study) and Europe (the CNIC and Amsterdam UMC). The goal of TANSNIP is to develop tools for lifestyle improvement based on the use of imaging techniques to detect the presence of atherosclerosis in its early stages, before the appearance of symptoms and events such as heart attack or stroke.
In 2015, the TANSNIP project launched a major intervention to promote a heart-healthy lifestyle in 1,000 individuals from the PESA-CNIC-Santander cohort in Madrid. The original goal of the PESA-CNIC-Santander study was to detect atherosclerosis long before the appearance of symptoms and thus to gain an understanding of the factors that trigger its development and progression.
“After months of work with our partners at Amsterdam UMC and with Banco Santander’s medical services, we designed a lifestyle intervention for participants who are part of the PESA-CNIC-Santander cohort,” explained Dr. Inés García-Lunar, a cardiologist at the CNIC and first author of the study.
The intervention consisted of a program of 12 motivational sessions distributed over three years in which an expert psychologist provided participants with the tools to introduce heart-healthy changes into their lifestyle. Participants also were given a physical activity bracelet to monitor the number of steps per day and a sit-stand desktop that allowed them to alternate sitting and standing during working hours, thus reducing sedentary time.
“More than 1,000 PESA-CNIC-Santander study participants at Banco Santander were randomly assigned to follow the intervention during their working hours or to continue with their normal routines,” said Borja Ibáñez, CNIC scientific director and a cardiologist at Fundación Jiménez Díaz University Hospital.
“We found that individuals assigned to the intervention increased their level of physical activity, improved their diet, and reduced their sedentary time. And as a consequence of these behavioral changes, these participants’ blood pressure and cholesterol also decreased,” explained Dr. José María Castellano, a cardiologist at the CNIC and scientific director of the HM Hospitales Research Foundation.
“A very important result is that the effect of the intervention decreases over time, which suggests that programs of this type need even more frequent reinforcement to achieve sustained changes,” noted Dr. Inés García-Lunar.
“The study represents a major finding, due to the complexity of implementing a program of these characteristics in a work environment, and this has been possible thanks to the hard work and commitment of all those involved, including the willing engagement of the participants,” explained Dr. Valentín Fuster, who is the principal investigator on the study.
TANSNIP-PESA is funded by Fundación Centro Nacional de Investigaciones Cardiovasculares (CNIC) Carlos III through an Investigator-initiated Study grant to Icahn School of Medicine from AstraZeneca. The PESA study is cofunded by the CNIC and Banco Santander. The study also received funding from the Carlos III Institute of Health and the European Regional Development Fund.