Señalización de los Receptores Nucleares
Our group investigates the transcriptional and epigenetic control of immune and cardiovascular cells in homeostasis and disease, with a particular focus on the molecular mechanisms underlying the actions of nuclear receptors. We use a combination of macrophage-specific knockouts, mouse models of disease, in vivo imaging, and next-generation sequencing technologies combined with bioinformatics approaches.
Different macrophage populations express distinct sets of transcription factors that are refined by tissue-specific microenvironmental cues. There is currently no clear understanding of the signaling molecules and mechanisms that drive the differentiation and specialized functions of most tissue-resident and monocyte-derived macrophages. Our group has made important contributions to deciphering the role of retinoid X receptor (RXR) nuclear receptor both in the generation of specialized tissue-resident macrophage subsets in homeostasis and in the actions of monocyte-derived macrophages in inflammation and tissue injury. Ongoing studies in the lab are exploring the role of RXR in macrophage identity and function and in the control of myeloid cell differentiation from hematopoietic stem cells.
A key focus of our current work is deciphering the transcriptional and epigenetic mechanisms controlling tissue macrophage identity, origin, and function in the heart and defining how these mechanisms impact physiological and pathological responses in atherosclerosis, stroke, and myocardial infarction. We recently identified novel factors and epigenetic regulators in the injured heart and uncovered the transcriptional variety of heart macrophages after myocardial infarction. This work revealed the presence in the heart of distinct macrophage subsets with different reparative capacities, and we are currently exploring the roles of these factors and macrophage populations and how macrophages sense local environmental cues during cardiac repair.
Other work in the laboratory is aimed at elucidating how nuclear receptors control cardiomyocyte physiology. Cardiomyocytes are thought to be functionally and transcriptionally stable cells fully committed to contractile function. However, recent evidence suggests that the maintenance of cardiac function in health and disease depends on epigenetic regulation. We are particularly interested in the impact of RXR on chromatin remodeling and epigenetics in the heart during development, adulthood, and disease.
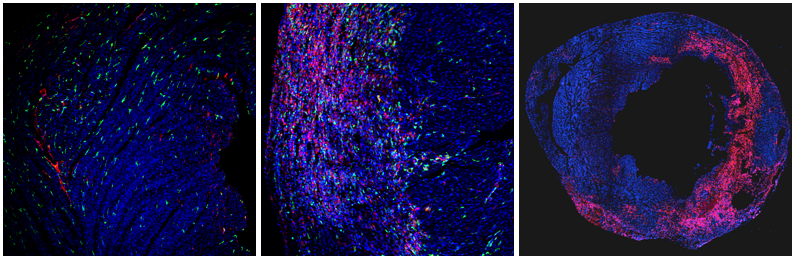
Our laboratory studies molecular mechanisms important in macrophage biology in a variety of physiological and pathological settings. The image shows cardiac macrophages (CD68 in red, CX3CR1 in green) in an uninjured heart (left) and at 3 (center) and 7 days post-injury (right).






