EXCELLENCE IN THE SCIENCE COMMUNICATION
The main scientific journals científicas publish research from the CNIC laboratories
Nature Communications
CNIC research identifies a key protein for ‘burning’ fat

A new study conducted at the CNIC and CNIO, led by Guadalupe Sabio and Cintia Folgueira, has uncovered a mechanism through which the body burns brown fat and converts it into heat. This process plays a protective role against obesity and related metabolic diseases.
The mechanism now identified is controlled by the protein called MCJ, present in mitochondria (the organelles of the cell where energy is produced). Sabio and Folgueira have discovered that, when the MCJ protein is removed from obese mice, these animals produce more heat and lose weight. The researchers have also managed to reduce the weight of obese mice just by having them transplanted with fat without that protein.
Obesity is the result of either excessive food intake or inadequate total energy expenditure. We now know that adipose tissue –body fat–, in addition to storing energy, plays a crucial role in the management of that energy by the body.
There are two types of fatty or adipose tissue: white and brown. White adipose tissue mostly stores energy, while brown fat (its cells have more mitochondria and that gives them a brown hue) is responsible for heat generation or thermogenesis, the process that maintains body temperature and which is triggered by cold or other stimuli.
The researchers also observed that “animals without MCJ in brown fat are protected against health problems caused by obesity, such as diabetes or increased blood lipids,” explain the two scientists. Therefore, they believe that the MCJ protein could be a new therapeutic target to correct diseases associated with obesity.
The research is now seeking to develop a therapy to block this protein in obese patients, but to do so they must first investigate whether the MCJ protein has vital functions in other tissues. At the same time, Guadalupe Sabio says, “we are trying to see if these changes in fat affect tumour growth or cachexia – loss of muscle and fat – which is also sometimes linked to cancer.”
Communications Biology
The European mitochondrial lineage may protect against severe forms of COVID-19
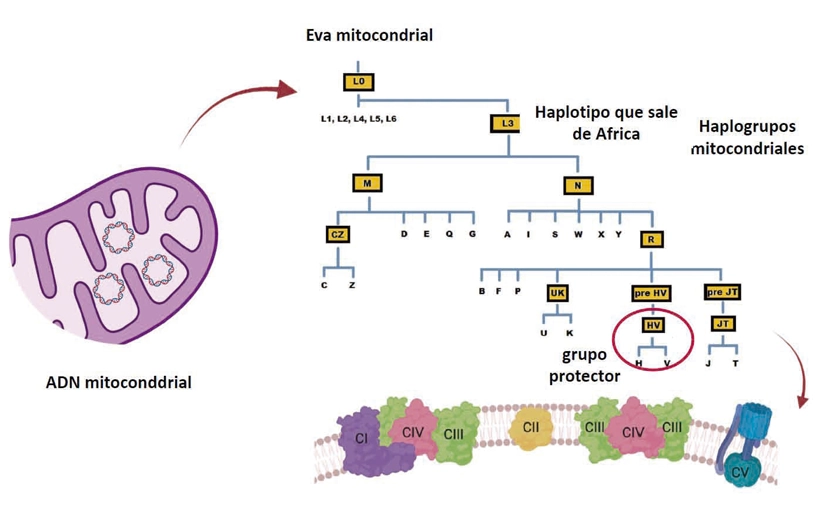
A study published in the journal Communications Biology shows that specific genetic variants in the mitochondrial DNA of Europeans may protect them against the more severe forms of COVID-19. The results of the study provide new clues to the origin of individual differences in the response to infection. The research was led by Dr. José Antonio Enríquez, head of the GENOXPHOS group at the CNIC and a member of the Spanish research network on frailty and healthy aging (CIBERFES).
The study identified patterns suggesting a possible relationship between certain mitochondrial haplogroups (genetic variants shared by individuals with a common ancestor) and susceptibility to more severe forms of COVID-19. “The study demonstrates the importance of the mitochondrial HV lineage as a protective factor against the severe consequences of infection with SARS-COV2, supporting the idea that mitochondria play a key role in the response to infectious disease,” explained José Antonio Enríquez.
These results advance our understanding of the variability in the response to SARS-CoV-2 and may help to improve the personalized care of patients with COVID-19.
The study analyzed data from more than 14300 patients in the SCOURGE registry (Spanish Coalition to Unlock Research on Host Genetics on COVID-19) and confirmed that individuals with genetic variants found in the HV lineage—the most common mitochondrial lineage among Europeans, accounting for 37%–58% of the European population–had a lower risk of developing severe forms of COVID-19. Mitochondrial DNA is the only part of the human genome that is inherited exclusively through the maternal line and plays a key role in the cellular generation of energy and the inflammatory response.
In addition to the CNIC and CIBERFES, acclaimed Spanish and international institutions participating in the study included the University of Santiago de Compostela (USC), Marqués de Valdecilla University Hospital–IDIVAL, Nuestra Señora de Candelaria University Hospital, La Paz University Hospital, and the Autonomous University of Nuevo León (México).
The study used advanced genetic analysis models to identify data patterns, revealing how previous pandemics may have influenced the current distribution of these genetic variants in Europe through the process of natural selection.
Immunity
CNIC scientists discover how the gut modulates the development of inflammatory conditions

A study led by David Sancho at CNIC in Madrid reveals how an increase in intestinal permeability allows the natural gut bacteria to cross the intestinal barrier and reach the bone marrow, where they induce epigenetic changes—modifications that alter gene activity without affecting DNA sequence—in the stem cells that give rise to immune cells. The epigenetic changes induced by the translocated gut bacteria generate “trained” immune cells primed to respond more efficiently to future infections. However, this same ability to amplify the immune response can also aggravate the inflammatory conditions such as cardiovascular and neurodegenerative diseases. The new study highlights the key role in this process of a protein called Mincle, expressed in cells of the innate immune system.
The study is published in the journal Immunity and was conducted in collaboration with research teams led by José Luis Subiza (Inmunotek S.L., Alcalá de Henares), Carlos del Fresno (IdiPaz, Madrid), Salvador Iborra (Universidad Complutense de Madrid) and Juan Duarte (Universidad de Granada).
Trained immunity explained David Sancho, who leads the Immunobiology lab at the CNIC, allows macrophages and other cells of the innate immune system to respond more efficiently to future encounters with bacteria, fungi, or viruses. “The protection this mechanism provides against viral and fungal infection has been demonstrated in animals with heightened intestinal permeability, which resulted in a stronger inflammatory response and greater resistance to infection.”
Until very recently, scientists believed that specific—or adaptive—immunity was the only type with memory, able to generate cells that ‘remember’ previous encounters with pathogens and unleash a specific immune response. In contrast, the innate immune response, which is not specific to a particular pathogen, was believed to lack memory. “We now know that innate immunity can be ‘trained’ to produce a stronger response to later, unrelated infections. What is more, the effects of this training are long-lasting,” explained Dr. Sancho.
The findings of the study open new routes to understanding the relationship between gut health and systemic diseases, underlining the importance of a healthy diet and a balanced microbiota as key elements in disease prevention.
The study was funded by the Spanish Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación (AEI); the European Union NextGenerationEU/PRTR; the Comunidad de Madrid; Fundación Científica de la Asociación Española Contra el Cáncer; Worldwide Cancer Research; European Research Council; Inmunotek S.L., y Fundación “la Caixa”.
JACC CardioOncology
CNIC scientists design an effective treatment strategy to prevent heart injury caused by a class of anticancer drugs

A team of scientists at CNIC, working in collaboration with international partners, has designed a strategy for preventing the cardiotoxic effects of anthracyclines, a widely used class of anticancer drugs. Cardiotoxicity is a frequent adverse secondary effect of cancer therapy with these drugs. The study, published in JACC: CardioOncology, demonstrates that treatment with the SGLT2 inhibitor empagliflozin can mitigate the cardiac injury associated with anthracycline therapy.
Using an advanced experimental model, the CNIC team showed that administration of a 20 mg daily dose of empagliflozin preserved the contractile function of the hearts of pigs treated with anthracyclines and protected the metabolism of the heart muscle. The protective effect of empagliflozin identified in the study involves an increase in the myocardial consumption of ketone bodies. This preserves the production of ATP (adenosine triphosphate, the main source of transferable energy in living cells) and mitochondrial function. “Treatment with empagliflozin protects the heart by directly addressing the metabolic processes affected by the cancer therapy,” said Dr. Ibáñez.
Our study demonstrates that empagliflozin prevents structural alterations in cardiomyocytes such as cellular atrophy and DNA damage. These results underline the potential of SGLT2 inhibitors not only in the treatment of heart failure, but also as a preventive therapy in cancer patients receiving treatments associated with severe cardiovascular side effects. The study, funded by the European Commission (ERC-CoG 819775), the Spanish Ministry of Science, Innovation and Universities, and the Community of Madrid regional government, opens the way to new clinical trials in patients at high risk.
Nature
CNIC scientists discover a type of immune cell that produces defensive “shields” in the skin

A team at the CNIC led by Dr. Andrés Hidalgo has discovered a specialized population of neutrophils in the skin that produce extracellular matrix, helping to maintain the skin’s resistance and integrity. The study, published in Nature, demonstrates that the immune system not only targets pathogens, but also physically strengthens the skin to prevent them entering the body.
Neutrophils are an important type of circulating immune cell. The specialized neutrophils described in the new study populate the skin, where they produce collagen and other matrix proteins that strengthen the skin barrier. The discovery broadens understanding of the immune system and may lead to new strategies for treating skin diseases, inflammation, diabetes, and age-related conditions.
Although neutrophils are best known for their microbicidal properties, the new study reveals an unexpected role for these cells in the generation and remodeling of the subepidermal extracellular matrix. The extracellular matrix is critical for maintaining the structure and function of the skin and other tissues, acting as a barrier to the entry of microorganisms and toxins.
The study, demonstrates that these neutrophils help to maintain skin integrity under normal conditions and are activated in response to injury to generate protective structures around wounds that prevent the entry of bacteria and toxins.
The study further shows that this structural function of skin neutrophils is regulated by the TGF-β signaling pathway. By genetically deleting this pathway in mice, the authors showed that the deposition of extracellular matrix was diminished, resulting in skin that was more fragile and permeable. Hidalgo notes that.
This suggests that the interaction between the immune system and the body’s structural components is much more direct than previously believed.
Another fascinating result emerging from the study is that the activity of these skin neutrophils follows a day-night pattern, adjusting the production of extracellular matrix according to the body’s circadian cycle. As a result, the skin of mice is more resistant at night than during the day, thanks to the nocturnal peak in neutrophil activity. Hidalgo underlines that, “This finding opens new avenues for investigating how internal body rhythms influence tissue defence, regeneration and repair.”
For Hidalgo, now at Yale University School of Medicine, the discovery of matrix-producing neutrophils not only broadens knowledge about innate immunity, but also suggests new treatment strategies for skin diseases and immunological disorders. He explains, “These findings will help develop treatments to strengthen the skin barrier in patients with inflammatory diseases or immunological alterations, including patients with diabetes and older adults.”
The study was supported by funding from Fundación “la Caixa”, the Boehringer Ingelheim Foundation, the National Institutes of Health, and the Swiss National Science Foundation.
NEJM
A drug used to treat diabetes improves the prognosis of patients undergoing cardiac valve intervention

A drug used to treat diabetes improves the prognosis of patients with aortic stenosis treated by transcatheter aortic valve implantation (TAVI). The results of a new clinical trial including 1,250 patients shows that medication with dapagliflozin after this treatment reduces the rates of death and hospital admission attributed to heart failure over a one-year follow-up. The trial was coordinated by the CNIC and Hospital Álvaro Cunqueiro de Vigo, and the study results are published in The New England Journal of Medicine.
Aortic stenosis is a degenerative disease that typically progresses slowly, narrowing the aortic valve opening through which blood is pumped from the heart to the rest of the body. As a result, the heart muscle has to work harder, and this can lead to heart failure, angina, or even sudden cardiac death due to malignant arrhythmias. Traditionally, this condition has been treated by heart valve replacement surgery. But the surgical risk for elderly patients is very high, and this limits their access to this intervention.
Before the new study, there were no treatments available to improve the prognosis of patients recovering after TAVI.
Both dapagliflozin and the related medication empagliflozin work by inhibiting sodium-glucose cotransporter 2 (SGLT2) and were originally developed as treatments for diabetes. However, they are also effective treatments for heart failure and have become key elements of the treatment of this condition. Despite the efficacy of these drugs, the mechanism through which SGLT2 inhibitors improve the prognosis of heart failure patients has remained a mystery. Dr. Borja Ibáñez—CNIC Scientific Director, cardiologist at Fundación Jiménez Díaz, group leader in the Spanish cardiovascular research network (CIBERCV), and the scientific coordinator of the trial—explains that, “Given the medical importance of this question, we have worked on it extensively, and we recently demonstrated that these drugs increase the energetic capacity of the heart by altering how the myocardium [heart muscle] utilizes nutrients to generate energy.” That study was published recently in JACC: CardioOncology.
Although patients with aortic stenosis frequently progress to heart failure, they have generally been excluded from clinical trials investigating the benefits of SGLT2 inhibitors. Dr. Raposeiras adds that, “Those trials also included minimal representation of elderly patients.”
This is precisely what inspired Dr. Ibañez’s team at the CNIC to conduct a clinical trial in the specific population of elderly patients treated with TAVI.
DapaTAVI is an independent trial conducted in Spain with no funding from the pharmaceuticals industry at 39 Spanish hospitals. The trial included 1,250 patients with severe aortic stenosis who were recovering after TAVI and at high risk of heart failure. Half of the study participants were treated with dapagliflozin after the TAVI procedure, while the remaining half received standard care. Analysis at the end of one year of follow-up showed that the rates of death and hospital admission due to heart failure were significantly lower in the dapagliflozin-treated group.
Summarizing the findings, second principal investigator Dr. Ignacio Amat-Santos—an interventional cardiologist at Hospital Universitario de Valladolid and a CNIC research scientist—says that, “This study demonstrates that we can further improve the prognosis of patients treated with TAVI, an especially vulnerable patient group.” CNIC General Director Dr. Valentín Fuster, a coinvestigator on the trial, underlines that, “The results of DapaTAVI will have an enormous impact on a growing number of patients and will help to extend their life expectancy with improved quality of life.” “DapaTAVI,” notes Dr. Ibáñez, “is an integral part of the CNIC’s strategy begun seven years ago to lead pragmatic clinical trials with no pharmaceutical industry involvement and with the goal of transforming global clinical practice.” Dr. Xavier Rosselló, of hospital son Espases de Palma de Mallorca and a CNIC scientist, adds that “These clinical trials, managed from the CNIC Clinical Trials Coordination Unit, are a demonstration of Spanish scientific leadership, as well as the commitment of the Spanish medical community.”
The investigators conclude that DapaTAVI marks a breakthrough in cardiovascular research and could transform the treatment of these patients throughout the world.
The study received funding from the Carlos III Health Institute, the Spanish Society of Cardiology, the Galician Society of Cardiology, and the Castilla y León Regional Health Authority.
Circulation
The heart remembers: scientists describe how early-life cardiac injury in parents influences the development and function of the heart in the next generation

Scientists at the CNIC and the University of Bern have found that heart surgery in male mice early in life creates a “memory” passed down to the next generation. Published in Circulation, the study suggests that a parental history of heart surgery should be considered when evaluating cardiovascular health in descendants.
Stress during the first years of life can have effects that last into adulthood. Less is known, however, about the possible inheritance of the consequences of early-life stress by the next generation. Now, scientists at the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) and the University of Bern in Switzerland have discovered that heart injury early in life in one generation of mice triggers changes in cardiac function in their offspring. The study is published in the journal Circulation.
A family history of heart attack is known to influence an individual’s risk of cardiovascular disease. Moreover, the risk for offspring is greater if a parent experienced a heart attack earlier in life. Nevertheless, it has remained unclear if heart injury in a parent directly influences the cardiovascular system of the next generation. Almost 30,000 children require heart surgery every year in Europe, so exploring whether the “memory” of early-life cardiac injury can be transmitted to the next generation offers an important opportunity to increase our understanding of cardiovascular disease and to improve the collection of medical histories.
The new study, led by Nadia Mercader of the CNIC and the University of Bern, analyzed if experimentally induced cardiac injury in male mice could produce an inherited effect in the next generation. The results, explains study first author Benedetta Coppe, of the University of Bern, show that the offspring of fathers with early-life cardiac injury had altered heart function. “The offspring of injured fathers showed evidence of altered heart development, characterized by transient expansion of the left ventricle during the first weeks after birth. This surprised us, since the only difference between the newborns was that in one group the father had experienced cardiac injury early in life, while in the other group the father was uninjured.” The offspring of injured fathers also showed alterations in their responses to cardiac injury. These changes included improvements in cardiac remodeling (changes in the size, shape, and function of the heart after induced injury) compared with the offspring of uninjured fathers. This superior cardiac remodeling was associated with an increased volume of blood ejected by the heart per minute.
After injury, the heart normally switches its energy source from lipids to glucose, and this results in an accumulation of lipids in the heart tissue. Dr. Mercader explains that, “Curiously, the offspring of injured fathers accumulated fewer lipids in response to induced heart injury and had higher concentrations of circulating lipids in the blood. These observations suggest that the metabolism of mice with this ‘family history’ recovers better when these mice are themselves subjected to cardiac injury.” The changes observed in the offspring of injured fathers indicate that cardiac surgery in the first weeks after birth leaves a lasting “memory” that can eventually be transmitted to the next generation. The researchers conclude that the findings open the way to a better understanding of the impact of heart disorders and underline the potential value of including family surgical history when collecting patient medical histories. The study was supported by funding from the European Union Horizon 2020 Programme (grant 819719) and an interdisciplinary grant (UniBeAQ20 ID Grant) from the University of Bern.
Circulation Research
Breakthrough gene therapy offers hope for rare, deadly heart disease in young men

A team at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) has developed an innovative gene-therapy strategy that could transform the treatment of arrhythmogenic right ventricular cardiomyopathy type 5 (ARVC5), a rare and highly penetrant inherited cardiac disorder with an elevated risk of sudden cardiac death. This disease is particularly devastating in young men and lacks a cure, with current treatments focusing on palliative care.
The study, led by Dr. Enrique Lara-Pezzi, head of the Molecular Regulation of Heart Failure group at the CNIC and a member of the Spanish cardiovascular research network (CIBERCV), demonstrates that the introduction of a healthy version of the TMEM43 gene directly into cardiac muscle cells significantly improves heart function and prolongs survival in a mouse model of the disease.
The new method is the result of more than 10 years of collaboration between a clinical team at Hospital Puerta de Hierro Majadahonda led by Dr. Pablo García-Pavía, who also heads the Inherited Cardiomyopathies group at the CNIC, and the basic–translational group led by Dr. Lara-Pezzi. This longstanding partnership has allowed the team to advance the understanding of this disease and offer an effective alternative for its treatment. After the identification of the first patients in Spain with ARVC5 at Hospital Puerta de Hierro, the two groups worked together to create, in 2019, the first model to replicate the disease in animals. Now, the researchers have taken a step further by developing a treatment for the disease in this experimental model.ARVC5 is caused by mutations in a gene called TMEM43 that provoke severe arrhythmias and can cause sudden death. The disease is particularly aggressive in young men, reducing their life expectancy to below 42 years. While implantable cardioverter-defibrillators (ICDs) are used to prevent sudden death, there are no available treatments to stem progression of the disease. In the study, published in Circulation Research, the CNIC team developed a gene therapy using adeno-associated viruses (AAV)—a safe delivery system for human patients—to deliver a functional copy of TMEM43 directly into the cardiac muscle cells of the experimental mice.
The results are promising: the treatment not only improved cardiac contraction and reduced fibrosis, but also significantly prolonged the life of mice with ARVC5-like disease. A single dose of the treatment was enough to prevent the electrical and structural alterations typical of the disease.
First author Dr. Laura Lalaguna explains that, “This advance takes us closer to a possible cure for this devastating disease. By increasing the amount of functional TMEM43 protein in the heart, we were able to counteract the toxic effects of the mutant version of the protein, and this resulted in improved heart function and put a brake on disease progression.” Treatments for heart failure associated with hereditary cardiomyopathies are often ineffective, and Dr. Lara-Pezzi is keen to underline the potential application of the new strategy in the treatment of other diseases of this type. “AAV gene therapy has enormous potential to offer specific solutions and cures not only for ARVC5, but also for other inherited cardiac disorders.” The study marks a key advance in the search for more effective treatments for rare diseases and could transform the prognosis of affected patients, alleviating the burden of hereditary cardiac diseases and reducing the need for continuous medical surveillance, to the benefit of both patients and health care systems.
The study was supported by the Pathfinder Cardiogenomics program of the European Innovation Council (project DCM-NEXT; 101115416); grants PID2021-124629OB-I00, TED2021-129774B-C22, and PLEC2022-009235 from the Ministerio de Ciencia e Innovación (MCIN/ AEI/10.13039/501100011033); the NextGenerationEU recovery instrument through the PRTR (Plan de Recuperación, Transformación y Resiliencia de España); and the European Regional Development Fund.
Nature Communications
A type of immune cell strengthens immunotherapy and prevents tumor relapse in animal models

Scientists at the CNIC, working in collaboration with the Instituto de Investigación Biomédica de Barcelona (IRB Barcelona), have discovered a new immunotherapy strategy that reduces cancer recurrence in mouse experimental models. The study, published in Nature Communications, shows that a specific subtype of immune cell—type I dendritic cells— is especially effective at activating a strong immune response and generating immune memory against cancer.
Dendritic cells act as sentinels of the immune system, presenting tumor antigens to T lymphocytes to initiate an immune response against malignant cells. However, there are multiple dendritic cell subtypes, and until now it was unclear which is the most efficient at generating a sustained, protective response to tumors.
First author Ignacio Heras-Murillo, of the CNIC, explains: “The finding is important because most current therapies focus on enhancing the immune response that is already in place. In contrast, this dendritic cell-based immunotherapy aims to induce a new, specific immune response against the tumor.”
Describing the strategy, study co-supervisor Stefanie Wculek—formerly at the CNIC and now at IRB Barcelona—explains that dendritic cells are extracted from the cancer-bearing mouse, loaded in the lab with tumor-derived antigens, and then reintroduced into the animal to activate specific T lymphocytes. The results show that type I dendritic cells not only trigger an immediate response against the primary tumor but also induce long-lasting immune memory capable of preventing tumor relapse. CNIC scientist David Sancho, who led the study, adds that immunotherapy with type I dendritic cells presenting the tumor antigen triggers an immune memory response that prevents the growth of a second, similar tumor. In other words, the treatment helps prevent relapse in the mouse models. Further studies will be needed to assess the potential of this approach for preventing metastasis and its possible synergy with other antitumor therapies.
The study was supported with funding from the CNIC; the Ministerio de Ciencia, Innovación y Universidades (MICIU), the Agencia Estatal de Investigación, the European Union NextGenerationEU/PRTR; the Comunidad de Madrid; the “la Caixa” Foundation; Asociación Española Contra el Cáncer, and Worldwide Cancer Research (25-0080).
Developmental Cell
CNIC team reveals how the heart is organized from the earliest stages of embryonic development

A study published today in the journal Developmental Cell uncovers new insights into how the heart forms during the earliest stages of embryonic development. The research, led by scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), shows that the heart originates from two distinct cell populations that form independently—but in a coordinated manner—from the very beginning of development: specifically, from the onset of gastrulation, the process by which the embryo begins to organize its basic cellular layers.
“This discovery is highly significant,” says CNIC scientist Miguel Torres, head of the Genetic Control of Organ Development and Regeneration Group and senior author of the study together with Miquel Sendra. “It helps us understand how the heart is structured in its earliest phases, which may allow us to identify the roots of certain congenital heart defects. It also opens new doors for regenerative medicine and tissue bioengineering.”
Until now, it was thought that cardiomyocytes—the muscle cells of the heart—and endocardial endothelial cells—which line the heart’s inner surfaces—originated from a single precursor population.
However, the new study, based on in vitro mouse embryo culture, advanced microscopy, and cell tracing techniques, reveals that these two cell types originate from separate regions within the mesoderm, one of the three germ layers of the early embryo.
Although they develop separately, the two cell populations enter the embryo simultaneously and migrate in a coordinated fashion toward the region where the primitive heart tube begins to form. According to the researchers, this synchronized behavior points to the presence of highly precise organizational mechanisms at a stage when the embryo still lacks most visible structures.
The study also shows that, despite being fated to form the heart, these cells retain the potential to contribute to the development of other organs—highlighting their versatility and importance in early embryogenesis.
The study was funded by the “la Caixa” Foundation (ID 100010434); the Company of Biologists; the Spanish State Research Agency; the European Commission’s H2020 program REANIMA, and CARDIOBOOST-CM of the Community of Madrid.
It also received support from the ERDF ‘A way to build Europe’ (ReDIB ICTS TRIMA@CNIC infrastructure, MCIN).
Circulation Research
CNIC scientists uncover how the immune response drives atherosclerosis—the root cause of heart attacks and strokes

Scientists at the CNIC in collaboration with national and international research centers, have identified a key immune cell subtype involved in the development of atherosclerosis. In a study published in Circulation Research, the team tested an experimental therapy in animal models based on immunosuppressive nanoparticles and demonstrated that it can slow disease progression.
The new CNIC research, conducted by the Immunobiology Group led by David Sancho, reveals that conventional type 1 dendritic cells (cDC1s) play a key role in this inflammatory process. The team used mouse models to analyze how the presence or absence of these cells influences atherosclerosis development. “We used genetically modified mice fed a high-cholesterol diet to replicate the conditions that promote atherosclerosis,” explains lead author Miguel Galán Burgos. “When we artificially increased the number of cDC1s, arterial lesions worsened. But when the mice lacked cDC1s specifically, plaque formation was significantly reduced—even with an unhealthy diet.”
A key innovation in the study was the development of an experimental therapy based on nanoparticles loaded with the immunosuppressant dexamethasone and coated with antibodies. These nanoparticles were developed in collaboration with Jesús Ruiz Cabello and Susana Carregal Romero, from the Molecular and Functional Biomarkers research group of the Center for Cooperative Research in Biomaterials (CIC biomaGUNE) in San Sebastian.
The nanoparticles were specifically designed to target cDC1s. “When we administered the nanoparticles in animal models of atherosclerosis, we observed a marked reduction in plaque size and in the associated inflammatory response. Importantly, this approach controlled arterial inflammation without impairing the body’s ability to fight viral infections”, explain the authors.
The results reinforce the role of cDC1s in driving atherosclerosis and introduce a promising new therapeutic approach that tackles the immune root of the disease. By precisely targeting the immune cells involved, this strategy could offer a safer, more effective alternative to current treatments, minimizing systemic side effects.
The project was supported by funding from the Spanish Ministry of Science, Innovation, and Universities (MICIU): PID2022-137712OB-I00, PID2021-123238OB-I00, PID2022-139218OB-I00, CNS2023-143944, RYC2020-030241-I, PID2022-142842OB-I00, CPP2021-008310 and CPP2022-009762, funded by the State Research Agency and the European Union NextGeneration EU/PRTR. Additional funding was provided by the Community of Madrid (P2022/BMD-7333 INMUNOVAR-CM) and Fundación “la Caixa” (LCF/PR/HR23/52430012 and LCF/PR/HR22/52420019).
Nature Biomedical Engineering
Descubren un método para estudiar proteínas mecánicas y su relación con enfermedades musculares

A team at the CNIC, led by Dr. Jorge Alegre-Cebollada, has developed an innovative method, called TEVs-TTN, for studying the specific mechanical functions of proteins through their controlled cleavage, a process that renders the proteins unable to sense and transmit mechanical force. The study results extend knowledge about the development of muscular diseases.
The study, published in Nature Biomedical Engineering, demonstrates that interrupting mechanical transmission by the protein titin precipitates muscular diseases. This finding opens new routes to understanding muscular dystrophies and other diseases associated with the protein titin.
Titin is the largest protein in animals and plays a critical role as the structural linchpin of sarcomeres, the contractile units of muscle cells.
Mutations in the titin gene (TTN) are a leading cause of congenital muscular diseases and cardiomyopathies, explains first author Dr. Roberto Silva-Rojas.
“In the absence of experimental animal models with titin-cleavage mutations, our approach allows a structured and targeted analysis of the impact of these types of alterations. This makes TEVs-TTN an ideal tool for testing therapies designed to mitigate the effects of impaired sarcomere integrity.” One intriguing finding of the study is that titin cleavage caused complete disintegration of sarcomeres over the course of a few days, leaving muscle cells devoid of their basic functional unit. Nevertheless, these cells survived, suggesting that similar processes might operate in other situations, such as muscle tears, heart failure, or cardiotoxicity associated with chemotherapy.
The methodology developed at the CNIC marks a milestone in the study of how protein mechanics contribute to tissue and organ physiology. Just as titin is critical for force transmission in sarcomeres, other proteins, such as dystrophin, dystroglycan complexes, integrins, and lamins, play critical roles in extracellular matrix regulation and cell membrane integrity. The new tool will enable the research team to confirm or refute hypotheses about the functioning of these proteins. These advances, in turn, could pave the way to the development of new therapeutic strategies for many diseases beyond those affecting muscle.
The study was funded mainly by the European Resarch Council through the ProtMechanics-Life Consolidator Grant (101002927) and a postdoctoral fellowship from the European Molecular Biology Organization to Dr. Silva-Rojas (EMBO ALTF 417-2022).













