1. FOREWORD AND CNIC MISSION
Valentín Fuster
General DirectorVicente Andrés
Basic Research DirectorBorja Ibáñez
Clinical Research DirectorThe Centro Nacional de Investigaciones Cardiovasculares (CNIC) is a biomedical research center funded through a pioneering public-private partnership between the Spanish Government and the ProCNIC Foundation (composed of 12 Spanish companies unrelated to the biomedical sector). The CNIC also benefits from the external support of its Scientific Advisory Board, composed of leading international experts who provide guidance on strategy and regularly evaluate the performance of the Center and its group leaders.
Cardiovascular disease (CVD) is the principal cause of death worldwide. The exponential increase in costs of the treatment of CVD in its symptomatic phase is creating an unaffordable burden on individuals and health systems. The CNIC’s mission is to improve cardiovascular (CV) health by advancing scientific knowledge and its effective transfer to clinical applications.
CNIC is organized to maximize collaboration between basic and clinical researchers and to encourage networking with hospitals. Thirty-one research groups (seven of which are led by clinicians) are distributed in three scientific areas: Myocardial Pathophysiology, Vascular Pathophysiology, and Cellular and Developmental Biology.
The CNIC is equipped with a state-of-the-art infraestructure that includes unrivalled advanced imaging technology recognized by the Spanish government as a national Unique Scientific and Technical Infrastructure (ICTS) and dedicated to the transmission and preservation of knowledge, technology transfer, and innovation.
At the end of 2020, the CNIC had a total staff in the Research Areas of 415 (64% women), with 106 predoctoral researchers and trainees (70% women). The CNIC is among the Spanish centers with the highest training capacity. Training at the CNIC is structured into 12 programs, including dedicated programs for clinical CV researchers.
In response to the worldwide COVID-19 pandemic in 2020, the Center kept its research running by promoting teleworking and maintaining laboratory-based work in accordance with the preventive measures established by Spanish health authorities.
This report offers an overview of how the CNIC adapted to this situation and how its energetic team of dedicated scientists, clinicians, technicians, and support personnel is bringing the CNIC’s mission to reality.
Major discoveries in 2020 include identifying a new origin of lymphatic vessels of the heart and of arteries, discovering new diagnostic and therapeutic targets for CVD and new functions of neutrophils, and the use of anticoagulants to potentially improve survival among hospitalized COVID-19 patients.
The Center’s ten large translational studies, including several randomized clinical trials, have already changed clinical practice worldwide. These studies bear testimony to the enthusiastic participation of researchers, healthy volunteers, patients, and emergency service personnel to defining the causes and risk factors of CVD. This commitment of citizens and professionals outside the research community is making essential contributions to advancing the use of noninvasive imaging technology for diagnosis and research.
Through these endeavors, the CNIC is making a comprehensive, across-the-board investment for societal benefit that integrates biomedical research into the wider society. This is fitting, since we are all stakeholders in our health and in the health of the next generation. As we move forward, the CNIC will maintain the drive and focus established in its initial phases and ensure that the Center’s basic and clinical scientists continue to work closely together to devise innovative projects that help reduce the health and socioeconomic burden associated with CVD, and to train the researchers of the future.
2. Research at the Center
The CNIC is organized into two departments, one focused on Basis Research and the other on Clinical Research.Research in these fields is fully interconnected through three multidisciplinary Research Areas.
2.1 Vascular Pathophysiology
Almudena R. Ramiro
CoordinatorScientists in the Vascular Pathophysiology Area (VPA) investigate the biology of the vascular system in health and disease through multidisciplinary approaches that include molecular and cellular biology, animal models of disease, and translational and clinical studies. This research takes advantage of high-throughput genomics, proteomics, and metabolomics coupled to bioinformatics analysis, as well as state-of-the art imaging technologies. The area hosts 12 research groups and 3 core technical units. The VPA has a particular interest in vascular biology and atherosclerosis, the underlying cause of heart attack and stroke.
Scientific highlights in 2020 include the following:
- By using mouse models of aortic disease and various complementary approaches, including non-invasive imaging, lentivirus-mediated transduction of the aorta, transcriptomics, and proteomics, we have identified novel pathogenic mediators and signaling pathways in diseases that involve vascular wall remodeling, including syndromic and non-syndromic aortic diseases and hypertension. Some of the identified mediators in mice have been validated in the human disease and are potential candidate therapeutic targets and disease biomarkers.
- Using several animal models that recapitulate vascular cognitive and mixed Alzheimer’s disease/vascular dementia pathology, we are unraveling mechanisms trough which cardiovascular risk factors affect cognitive function, and have identified novel prognostic and therapeutic targets related to the role of adult hippocampal neurogenesis in this setting.
- Through a single-cell proteomics analysis of the atherosclerosis antibody repertoire, we have identified a novel atherosclerosis antigen that has potential as a biomarker and a therapeutic target.
- High-throughput proteomics revealed that activation of the complement system is a major alteration in early atherosclerotic plaques and that elevated plasma C5 is a promising biomarker of subclinical atherosclerosis.
- We identified mechanisms that precipitate cardiovascular disease progression and aging in Hutchinson-Gilford progeria syndrome (HGPS). High-throughput proteomics identified common cardiometabolic alterations and dysregulated pathways in mouse and pig models of aging, and we also established a new mouse model of HGPS exhibiting premature vascular aging and features of atherosclerotic plaque vulnerability.
- The Genomics Unit successfully implemented an in-house method for the sensitive detection of ultra-low variant allele frequency (VAF) mutations. This strategy has allowed us to identify and monitor clonal hematopoiesis events associated with cardiovascular risk. Additionally, last year saw a surge in single-cell transcriptomics experiments, in which tens of thousands of cells are analyzed in a single experiment. scRNA-Seq data can be analyzed and integrated with public data in an ad-hoc tool developed in-house and available at https://bioinfo.cnic.es/scdavis/. This tool allows visualization and integrative analysis of single-cell transcriptomics and flow-cytometry data.
- Research into tissue and organismal aging identified a stem cell subpopulation with superior muscle regenerative capacity. We also found that sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals, shedding light on the emergence and remodeling of nestin-expressing coronary vessels. Work in 2020 also targeted cellular senescence to reverse regeneration failure in aging and diseases of the heart and skeletal muscle.
- Finally, we studied the molecular mechanisms that control cardiac chamber development and how are they altered in certain cardiomyopathies. We also characterized the interaction between the actin cytoskeleton and the various signals that govern the early steps of chamber formation, and studied how cardiomyocyte stiffness can lead to cardiac functional decline. Moreover, we have identified a gene signature in peripheral blood that predicts aortic valve calcification, and we are currently trying to expand this gene profile.
TECHNICAL UNITS
RESEARCH GROUPS
Molecular and Genetic
Cardiovascular Pathophysiology
Vicente Andrés
Experimental Pathology of
Atherosclerosis
Jacob Fog Bentzon
Intercellular Signaling in
Cardiovascular
Development and Disease
José Luis de la Pompa
Cardiovascular Imaging
and Population Studies
Valentín Fuster
Hematovascular
Pathophysiology
José Javier Fuster
Regulatory Molecules
of Inflammatory
Processes
Pilar Martín
Neurovascular
Pathophysiology
María Angeles Moro
Tissue
Regeneration
Pura Muñoz
B Lymphocyte
Biology
Almudena R. Ramiro
Gene regulation in Cardiovascular Remodelling and Inflammation
Juan Miguel Redondo
Intercellular Communication
in the
Inflammatory Response
Francisco Sánchez Madrid
Cardiovascular
Proteomics
Jesús Vázquez
2.2 Myocardial Pathophysiology
Guadalupe Sabio
CoordinatorThe Myocardial Pathophysiology Area (MPA) includes 11 research groups and 5 core technical units (Transgenesis, Pluripotent Cell Technology, Comparative Medicine, Viral Vectors, and Clinical Trials Coordination). MPA groups work on a wide range of topics: inherited cardiomyopathies, arrhythmia mechanisms and therapy, molecular regulation of heart failure, metabolism and its effect on cardiovascular diseases, functional genetics of the oxidative phosphorylation system, translational cardiovascular imaging and therapy, molecular cardiology, immunobiology, cardiovascular health and imaging, cardiac arrhythmias, and nuclear receptor signaling. Research in these areas produced several scientific advances during 2020.
- Results from a CNIC-led multicenter showed that cardiac electrical signals from patients fitted with pacemakers or implantable cardioverter defibrillators can be used to monitor and predict atrial remodeling progression in a personalized and patient-specific manner. This can be achieved with standard data transmission technology installed in the implantable device. (Jose María Lillo-Castellano et al. Europace 2020;22:704-715).
- As part of the PESA project, we designed an algorithm that provides a personalized estimate of cardiovascular risk in healthy middle-aged individuals based on a range of variables including age, blood pressure, diet, and blood and urine markers. The EN-PESA algorithm is an affordable tool for estimating the severity of subclinical atherosclerosis—characterized by the deposition of fatty substances in the arterial walls—especially in individuals at higher risk. We believe that EN-PESA will help to personalize the estimation of cardiovascular risk, leading to tailored treatments and follow-up plans.
- Dysregulation of the circadian clock has been linked to cardiometabolic disorders. We found that neutrophils regulate the liver circadian clock and also affect liver metabolism. Regulation of the liver’s molecular clock may provide a new therapeutic approach for metabolic diseases such as diabetes and high blood pressure. We also demonstrated that changes in cholesterol and bile acid metabolism promote cholestasis, bile duct proliferation, and intrahepatic cholangiocarcinoma.
- We found an alarmingly low prevalence of ideal cardiovascular health among early adolescents in Spain. This study demonstrated that adherence to lifestyle interventions can improve health outcomes in people living in diverse and socioeconomically disadvantaged communities.
- To investigate the molecular mechanisms underlying arrhythmias and sudden cardiac death in patients with Andersen-Tawil Syndrome and Short-QT syndrome, we generated mouse models with cardiac-specific expression of mutant Kir2.1 channels, as well as patient-specific induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). Our approach is multidisciplinary, involving virus-mediated gene transfer in mice, generation of iPSC-CMs from somatic cells obtained from patients carrying a mutant gene, in-silico modeling of ion channel structure-function relations, transcriptomics, protein chemistry, patch-clamping, ECG recordings, intracardiac stimulation, and optical mapping.
- We have shown how macrophages communicate with endothelial cells to promote cardiac repair and remodeling. We demonstrated that macrophages promote endothelial-to-mesenchyme transition after myocardial infarction and showed that macrophages and the metalloproteinase MMP14 are key regulators of this process. These findings indicate that patients at risk of developing heart failure after myocardial infarction could benefit from treatments based on controlling dysregulated myocardial MMP14 activity in macrophages with specific MMP14 inhibitors and the implementation of novel nanotechnology-based tools for selective macrophage targeting.
- In the cardio-oncology field, it is important to highlight the H2020-HEALTH Project. The CNIC-coordinated project Remote Ischemic Conditioning in Lymphoma Patients Receiving Anthracyclines (RESILIENCE, €6 million) involves 11 partners from 6 European countries. Moreover, through the ongoing ERC-CoG project Novel mitochondria-targeting therapies for chemotherapy-induced cardiotoxicity (MATRIX), we discovered a new mitochondria-targeted therapy for anthracycline-induced cardiotoxicity. Thanks to the use of a large animal model, we identified remote ischemic conditioning as a strong protective intervention (Cardiovasc Res. 2020;117:1132-1143). This therapy is now being tested in a randomized clinical trial (MATRIX).
- In the ischemia–reperfusion field, we identified metoprolol as the only beta blocker able to abrogate infarct-related exacerbated inflammation (Eur Heart J 2020;41:4425-4440). Also in 2020, the REBOOT trial reached half of the anticipated sample size (4250 of 8500).
- The MADRID-COVID trial was conducted in 2020. This trial tested the benefits of metoprolol in critically ill covid19 patients on mechanical ventilation. The results of the trial will be reported soon.
The MPA’s core technical units give support to all CNIC scientists:
- The Transgenesis Unit provides services in mouse strain rederivation, production of genetically modified mice, and cryopreservation of mouse strains. Through collaboration with Dr. Jorge Nicolás Domínguez Macías and Dr. Miguel Torres, we are currently developing a microinjection model in post-implantation embryos, which will allow us to obtain interesting results.
- The Pluripotent Cell Technology Unit Pluripotent Cell Technology Unit (PCTUnit) provides technological support in the generation of biological models, both in vivo and in vitro, through the manipulation of mouse embryonic stem cells (mESCs) and human induced pluripotent cells (hiPSCs). In 2020 the Unit collaborated with CNIC researchers on the derivation of hiPSCs from patient-derived dermal fibroblasts, helped fine-tune differentiation protocols for obtaining hiPSC-derived cardiomyocytes, and designed CRISPR/Cas9-based gene editing strategies for generating mouse and pig models and for in vitro CVD modeling in hiPSCs.
- The Clinical Trials Coordination Unit has continued to provide specialized support for clinical trials and studies carried out at the CNIC. Its ultimate goal is to boost Spanish leadership in clinical trials in the cardiovascular area.
- The Viral Vectors Unit provides researchers with access to state-of-the-art viral vector technology for use in preclinical studies and basic research.
- The Comparative Medicine Unit supports in vivo work in the animal facility.
TECHNICAL UNITS
RESEARCH GROUPS
Inherited
cardiomyopathies
Juan A. Bernal
Functional Genetics of the Oxidative
Phosphorilation System (GENOPHOS)
José Antonio Enríquez
Cardiovascular Health
and Imaging
Rodrigo Fernández
Advanced Development
in Arrhythmia Mechanisms and Therapy
David Filgueiras
Translational Laboratory
for Cardiovascular Imaging and Therapy
Borja Ibáñez
Cardiac
Arrhythmia
José Jalife
Molecular Regulation
of Heart Failure
Enrique Lara-Pezzi
Molecular
Cardiology
Silvia Priori
Nuclear Receptor
Signaling
Mercedes Ricote
Stress kinases in Diabetes, Cancer
and Cardiovascular Disease
Guadalupe Sabio
2.3 Cell and deVeloPMental Biology
Jorge Alegre-Cebollada
CoordinatorThe Cell and Developmental Biology (CDB) area comprises 8 research groups and 3 core technical units (Microscopy, Flow Cytometry, and Advanced Imaging). CDB area members have broad interests in immunology, cell biology, development, biomechanics, nanotechnology, cardiology, and epidemiology, creating a highly nurturing environment for leading-edge interdisciplinary research spanning basic and translational cardiovascular physiology and disease.
Research highlights in 2020 include the discovery that cardiac resident macrophages, a type of immune cell, help cardiomyocytes to eliminate damaged mitochondria. We also found that neutrophils in different tissues adopt unique characteristics that allow them to perform tissue-specific functions, and that the activity of these immune cells fluctuates in a circadian manner. Other work identified mechanisms governed by membrane structures known as caveolae through which cells sense the mechanical properties of their environment and secrete non-collagen ECM proteins to remodel it. Our scientists also uncovered new systems controlling limb and vessel development.
These discoveries include a gradient of transcription factors along the proximo-distal axis of the developing limb that provides essential positional information, the unexpected contribution of cells derived from the second heart field to the coronary lymphatic vasculature, and novel molecular mechanisms sustaining artery development. An emerging research interest in the CDB area is myocardial regeneration, an important process that can limit and revert injury after a myocardial infarction (MI).
CBD scientists have engineered several animal models to decipher molecular and cellular mechanisms underlying heart regeneration, including a mouse model that makes it possible to mechanically phenotype titin, a key protein of the contractile apparatus of cardiomyocytes. From a clinical perspective, we have contributed to the development of quality indicators for the management of acute MI. Looking to the future, in 2020 we launched two coordinated international consortia. REANIMA aims to enable cardiac regeneration after MI, while AtheroConvergence will decipher the role of mechanical forces in atherosclerosis. Additional international funds obtained in 2020 include two ERC Consolidator grants—to develop novel tools to manipulate protein mechanics in living matter and to explore vasculature development—and a Myokardia-Myoseeds grant to uncover molecular mechanisms sustaining development of dilated cardiomyopathy, the most frequent cause of heart transplantation worldwide. The Microscopy technical unit associated with the CDB area has secured funding from the European Regional Development Fund (ERDF) to set up pioneering imaging approaches for complex ultrastructural studies within the National Infrastructure ReDIB network.
The CDB area is committed to public outreach and has contributed to the CNIC’s efforts to address the challenges posed by the Covid-19 pandemic. In 2020, we established high specificity and sensitivity ELISA assays to detect antibodies to SARS-CoV-2 in human serum samples, and our scientists engage actively in public dissemination events related to the pandemic.
TECHNICAL UNITS
RESEARCH GROUPS
Molecular Mechanics of
the Cardiovascular System
Jorge Alegre-Cebollada
Molecular Genetics of
Angiogenesis
Rui Benedito
Multidisciplinary Translational
Cardiovascular Research (MTCR)
Héctor Bueno
Mechanoadaptation and
Caveolae Biology
Miguel Ángel del Pozo
Imaging the Cardiovascular
Inflammation and the Immune Response
Andrés Hidalgo
Development of the epicardium
and its role during regeneration
Nadia Mercader
Nanomedicine and
Molecular Imaging
Carlos Pérez Medina
Genetic Control of
Organ Development and Regeneration
Miguel Torres
2.4 Clinical Studies

Early Detection of Subclinical Atherosclerosis, Progression and Cardiovascular Health
(PESA-Health-CNIC-Santander Study)
Principal investigator:
Valentín Fuster
The PESA-Health-CNIC-Santander study is the natural continuation of the long-term endeavor started in 2010 with the PESA Study, carried out by the CNIC in collaboration with Santander Bank. Within PESA-Health, the PESA participants enrolled in 2010 (4184 asymptomatic individuals between the ages of 40 and 55 years at enrollment) will be actively followed up over an additional 10 years.
The original aim of the study was to identify the presence of subclinical atherosclerosis (SA) long before symptoms appear and to understand the cues leading to its development and progression. PESA-Health will expand these objectives to new areas, such as the correlation of SA with Alzheimer’s and cognitive diseases, the acquisition of somatic mutations during aging, and the correlation of these mutations with increasing cardiovascular event rates and SA progression. PESA-Health will continue to take advantage of state-of-the-art imaging technologies, including 3D vascular ultrasound of the carotid arteries and aorta, coronary artery calcium quantification by computed tomography, cardiac magnetic resonance, AngioTC, NaF PET, PET-amyloid analysis, and biosampling for omics analysis.
PESA-Health is the CNIC’s flagship study, and several CNIC clinical and basic research groups participate in it. The PESA study is already making seminal contributions to our understanding of the origin and progression of atherosclerosis.
The PESA-Health-CNIC-Santander study welcomed its first participant in February 2020, taking advantage of the follow-up of the PESA cohort to continue and expand the scientific approaches performed.

Secondary Prevention of Cardiovascular Disease in the Elderly Population
(SECURE)
Principal investigator:
Valentín Fuster
Co- Principal investigator:
José Mª Castellano
Adherence to treatment after acute myocardial infarction (MI) is essential for efficient secondary prevention. Despite this, many post-MI patients abandon prescribed medication. To address this issue, CNIC researchers and FERRER laboratories developed a “polypill” including three key drugs prescribed to post-MI patients (aspirin, an ACE-inhibitor, and a statin). Having demonstrated that prescription of the CNIC Polypill significantly increases treatment adherence among post-MI patients (J Am Coll Cardiol. 2014; 64:2071-82), CNIC researchers are now leading a multinational randomized clinical trial supported by the H2020 program. The ongoing SECURE trial (trial identifier NCT02596126) has enrolled around 2500 patients soon after an MI and randomized them to standard treatment or a CNIC Polypill-based strategy. Patients will be followed-up for a minimum of 2 years, and the incidence of major cardiovascular events will be assessed. Trial enrollment was completed by the end of 2019, and the trial is expected to complete its follow-up phase by the end of October 2021.
This trial grant has been extended until December 2021, and publication of the results is expected by mid 2022.

TREatment with Beta-blockers after myOcardial infarction withOut reduced ejection fracTion
(REBOOT)
Principal investigator:
Borja Ibáñez
The prescription of beta-blockers to patients after an MI is based on evidence from trials performed in the pre-reperfusion era. While there is solid evidence for the benefit of these drugs in post-MI patients with reduced ejection fraction, evidence is lacking for patients with preserved ejection fraction. Despite this, more than 80% of post-MI patients in this category are prescribed beta-blockers for the rest of their lives. REBOOT is a multinational trial that will enroll 8468 post-MI patients with a left ventricular ejection fraction >40%. Patients will be randomized to beta-blocker therapy (type and dose decided by the attending physician) or to no treatment. The primary endpoint is the composite of all-cause death, reinfarction, or heart failure admission during 3-year follow-up. This trial is coordinated by the CNIC Clinical Trials Coordination Unit and is run in close collaboration with the Mario Negri Institute of Research in Milan. More than 75 hospitals in Spain and more than 25 in Italy are participating in this large-scale project, which will have a major impact on clinical practice.
The first patients were enrolled in October 2018, and 4070 had been recruited by the end of 2020. The first follow-up assessment has been completed in 80% of patients, and the second follow-up in 45%.

The TANSNIP-PESA randomized control trial:
a 30-month worksite-based lifestyle program to promote cardiovascular health in
middle-aged bank employees
(TANSNIP)
Principal investigator:
Valentín Fuster
Existing tools for characterizing atherosclerosis and determining the risk of its complications are inadequate. These deficiencies limit effective management across the spectrum of this disease, and therefore opportunities are lost for early, cost-effective interventions in sub-clinical disease, while high-risk populations with manifest disease are administered treatments almost indiscriminately. This leads to high ‘numbers needed-to-treat’ (NNT), unnecessary patient risk, wasted resources, and unsustainable costs for health care purchasers. In a relatively low-risk population (the PESA-CNIC cohort), we are studying whether a personalized worksite based lifestyle intervention driven by imaging data (2D and 3D-ultrasound of the carotid and iliofemoral arteries, and coronary artery calcification) results in changes in behavior, improved control of risk factors, and reduced progression of subclinical atherosclerosis plaque burden (SAPB). TANSNIP is a randomized control trial (RCT) including middle-aged bank employees from the PESA cohort stratified by SAPB (high SABP n=260; low SABP n= 590). Within each stratum, participants are randomized 1:1 to join a lifestyle program or receive standard care. The program consists of three elements: (1) 12 personalized lifestyle counseling sessions using motivational interviewing (MI) over a 30-month period; (2) a wrist-worn physical activity tracker, and (3) a sit-stand workstation. The primary outcome measure is a composite score of blood pressure (BP), physical activity, sedentary time, body weight, diet, and smoking (the adapted FUSTER-BEWAT score) measured at baseline and at 1-, 2-, and 3-year follow-up. Secondary outcomes are individual changes in lifestyle behaviors and specific changes in anthropometric measures, blood biomarkers, self-rated health, work-related outcomes (including work productivity and absenteeism), health care consumption, program process measures, and cost measures at different measurement points.
The expectation is that individual awareness of CVD risk stratification in the intervention group will lead to a reduction in the prevalence of CV risk factors related to lifestyle and an increase in physical activity compared with the control group. A second rationale is that the level of compliance with the comprehensive 3-year worksite-based lifestyle intervention will be higher among participants with a high imaging defined CV risk. Follow-up is now complete, and the TANSNIP-PESA Study is now in the analysis phase.

Athero-Brain. The Heart to Head STUDY
(H2H)
Principal investigator:
Valentín Fuster
Co- Principal investigator:
Héctor Bueno
There is increasing awareness of the association between atherosclerosis and cognitive function, but the mechanisms linking these processes are not fully understood. The Heart-to-Head (H2H) study is testing the hypothesis that extensive subclinical atherosclerosis is associated with subtle cognitive decline and beta-amyloid deposition in the brain. This transatlantic collaboration is framed within an agreement between the CNIC and Mount Sinai Hospital in New York and is led by CNIC General Director Valentin Fuster. In Spain, the H2H project is coordinated between the CNIC and 12 de Octubre Hospital. Other university hospitals (Fundación Jiménez Díaz, Clínico San Carlos, and Gregorio Marañón) participate in the project, which receives funding from the Carlos III Institute of Health through the Proyecto Integrado de Excelencia program. A total of 300 participants are undergoing extensive atherosclerosis phenotyping (multi-territory 3D vascular ultrasound and cardiac computed tomography) and thorough brain imaging (anatomical and functional magnetic resonance imaging and positron emission tomography (PET)-amyloid scan), as well as cognitive function testing. Follow-up visits finished in 2020, and the study is now in the analysis phase.

Multimodality myocardial tissue characterization in patients with significant valvular disease
(MRVALVE)
Principal investigator:
Borja Ibáñez
The consequences of valvular heart disease (VHD) on left ventricular (LV) dimensions, function, and tissue composition are important determinants in clinical decision-making. Current practice guidelines recommend surgical treatment for patients with significant valvular heart disease when symptoms develop or when LV remodeling or dysfunction occur. The most prevalent valvulopathies are aortic valve stenosis (AS) and mitral regurgitation (MR). Transition from asymptomatic to symptomatic disease or from normal LV dimensions and function to LV dilatation/hypertrophy (LVH) and dysfunction is determined by changes in tissue composition (predominantly cardiomyocyte death, extracellular volume expansion, and fibrosis). The current therapies for severe VHD are surgery or percutaneous valve repair or replacement, and the decision to intervene is based on the presence of symptoms and/or gross anatomical and functional LV involvement, evident as significant chamber dilatation or reduced ejection fraction. When these features appear, it is often too late for interventions to fully restore heart function. There is therefore a need for tools for the early detection of myocardial involvement in patients with asymptomatic VHD, thus enabling appropriate intervention before overt deterioration of heart function. Cardiac magnetic resonance (CMR) is the gold standard for anatomical and functional cardiac assessment, including the detection of focal areas of fibrosis by late gadolinium enhancement (LGE) after contrast-gadolinium administration. Moreover, highly accurate tissue characterization is available with recent CMR advances such as parametric T1/T2 mapping, absolute myocardial perfusion quantification, extracellular volume calculation (a surrogate of diffuse fibrosis), and tagging. The assessment of focal and diffuse fibrosis requires endovenous contrast. We will use gadolinium contrast agent, which has the maximal safety profile and is in routine clinical use. Assessment of diffuse fibrosis also requires a blood sample for determination of the hematocrit. For the study of active deformation of the LV myocardium, the best imaging modality is strain echocardiography, which can detect impaired multidirectional strain (active deformation) even when overall LV function is preserved. We will correlate the imaging data with functional data from the 6-minute walking test, which provides an objective assessment of functional exercise capacity. The amount and extent of calcium deposition in the coronary arteries and heart valves will be assessed by cardiac computed tomography, a noninvasive method that gives the calcium score, a diagnostic and prognostic tool in AS patients. This project will use a multimodality imaging approach (CMR plus strain echocardiography) to better characterize LV status in patients with significant VHD, whether AS (a paradigm of LV pressure overload) or MR (a paradigm of LV volume overload).
So far 57 patients has been recruited, and 30 have completed the one-year follow-up visit.

Treatment with β3 agonists in chronic pulmonary hypertension secondary to heart failure
(SPHERE- HF)
Principal investigator:
Ana García-Alvarez
Co- Principal investigator:
Valentín Fuster and Borja Ibáñez
Pulmonary hypertension (PH) secondary to left heart disease (group 2 PH) is the most common form of PH and currently lacks effective therapy. CNIC researchers have identified the β3 adrenergic receptor as a novel therapeutic target for this disease in a large animal model of PH (Basic Res Cardiol. 2016;111:49). The CNIC is currently leading a phase 2 clinical trial in which group 2 PH patients are randomized to standard therapy vs standard therapy plus a β3-selective agonist (trial identifier NCT02775539 and Nº EudraCT: 2016-002949-32). A total of 80 patients are being recruited in four Spanish hospitals and will be followed under treatment for 4 months. The study endpoints are pulmonary artery hemodynamics and the CMR profile.
Enrollment was extended up to 2020. Of the 80 recruited patients, 75 had completed the 4-month follow-up by the end of 2020.

Pulmonary vasculopathy in patients with advanced heart failure
(HPIC)
Principal Investigator Hospital 12 de Octubre:
Juan F. Delgado Jiménez
Co-Principal Investigator CNIC:
Borja Ibáñez
Pulmonary hypertension due to left heart disease is a pathophysiological and hemodynamic state found in a wide range of clinical conditions that affect left heart structures. Although the pulmonary circulation has traditionally received little attention, it is today a fundamental part of cardiological evaluation. The most important clinical factors in heart-failure patients are the presence of pulmonary hypertension and right ventricular function. These factors are also essential prognostic determinants and must be taken into account when making important therapeutic decisions. The pathophysiological process starts passively but later transforms into a reactive process. This reactive process has a reversible component, which can be treated with vasodilators, and fixed component, in which the underlying mechanism is congestive vasculopathy (essentially medial hypertrophy and pulmonary arterial intimal fibrosis). There is currently no specific therapy available for this type of pulmonary hypertension, and treatment is the same as for heart failure itself. Drugs known to be effective in pulmonary arterial hypertension have generally shown a neutral effect in clinical trials. This project is focused on the clinical development of a number of groups of pharmacological compounds that will enable us to make progress in the near future.
So far we have recruited 45 patients and recruitment is continuing.

S. Pocock (Left) and B. Ibáñez (Right)

3D ULTRA-FAST COMPREHENSIVE CARDIAC MAGNETIC RESONANCE STUDY (CARDIO-RESONANCIA MAGNÉTICA INTEGRAL 3D ULTRA-RAPIDA)
(DASH – CMR)
Principal Investigator:
Borja Ibáñez
FIS-DTS (Proyecto de Investigación en Salud modalidad Proyectos de Desarrollo Tecnológico en Salud Ref DTS17/00136.)
This study is divided into 2 separate substudy protocols:
- Myocardial study, which uses gadolinium contrast to study post-infarction patients, cardiomyopathy, and myocarditis.
- Study of coronary angiography with MRI without gadolinium contrast, for the study of patients undergoing coronary angio-CT.
MRI exams in this study have been conducted at Hospital Universitario de Salamanca (1.5 Tesla MRI) and with the new MRI equipment located at the CNIC. This project was funded by a grant from the ISCiii (FIS-Technological Development (FIS-DTS). During the first year, we validated and improved the MRI technology for the pig model and worked on making study reconstruction achievable in real time in the MRI console itself.
Enrolment was completed in 2020, and a total of 147 participants were recruited.

Novel mitochondria-targeted therapies for cancer treatment-induced cardiotoxicity
(MATRIX)
Principal Investigator:
Borja Ibáñez
ERC Consolidator Grant#819775
The MATRIX Project aims to develop new and innovative treatments for cardiac toxicity associated with some cancer treatments. MATRIX will be jointly run at the CNIC and Fundación Jiménez Díaz (FJD) University Hospital within a collaborative framework established in 2015 to study myocardial diseases. Great advances in the treatment of cancer—a disease with 4 million new diagnoses every year in Europe—sometimes come with a 'toll' to pay in the form of major adverse effects. One of the most common adverse effects is myocardial toxicity, which affects up to 25% of patients treated with the common anticancer drugs anthracyclines or trastuzumab. The cardiotoxic effects of these drugs can be very serious and condemn the cancer survivor to chronic heart failure or even death from this complication.
Cancer treatment-induced cardiotoxicity (CTiCT) can result in severe heart failure. The trade-off between cancer and chronic heart failure places an immense personal burden on patients, with physical and psychological consequences. Current therapies for CTiCT are suboptimal, featuring poor early detection algorithms and nonspecific heart failure treatments. Our recently published results and additional preliminary data indicate that CTiCT is associated with altered mitochondrial dynamics, triggering cardiomyocyte metabolic reprogramming. MATRIX adopts a holistic approach to tackling mitochondrial dysfunction in CTiCT. We propose that early-stage CTiCT could be reverted by metabolic reprogramming to shift mitochondrial substrate utilization. By refining a novel imaging-based algorithm recently developed by our group, we will achieve very early detection of myocardial damage in patients treated with commonly prescribed cancer therapies, long before clinically used parameters become abnormal. Such early detection, not available currently, is crucial for early therapeutic intervention. We also hypothesize that in end-stage CTiCT, mitochondrial dysfunction has passed a no-return point, and the failing heart will only be rescued by a strategy to replenish the myocardium with fresh healthy mitochondria. This can be achieved with the radical new therapeutic option of in-vivo mitochondrial transplant. The MATRIX project has broad translational potential, including a new therapeutic approach to a clinically relevant condition, the development of technology for early diagnosis, and advances in knowledge of basic disease mechanisms.
Patient recruitment began in 2020, and by the end of the year we had already hosted 12 participants.
3. Scientific Highlights
by Publication date
DEVELOPMENT CELL
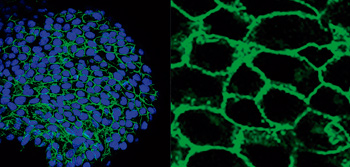
A CNIC-led international study discovers a new origin of lymphatic vessels in the heart
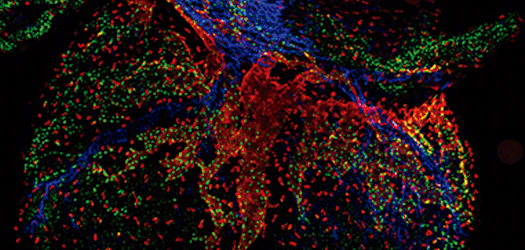
The study, published in Development Cell, has identified and characterized a new vasculogenic niche that contributes to the development of the cardiac lymphatic system. The study shows that the coronary lymphatic vessels have varied origins and functions: the results of the study reveal that the coronary lymphatic vasculature does not have a single origin, but instead forms through the participation of cells from different tissues. This study opens the way to future research into the mechanism underlying lymphatic vasculogenesis in this new niche and the functional diversity of coronary lymphatics.
This international study, led by Dr Miguel Torres’s group at the CNIC, examined the origin of the coronary lymphatic system during the formation of the heart in the mouse embryo. The study shows that the heart contains a second population of lymphatic cells that is recruited later during development and is derived not from veins, but from a region called the second heart field.
The second heart field is composed of multipotent cells “able to generate different types of heart cells, including cardiomyocytes (the cells of the cardiac muscle), smooth muscle cells, and the endothelial cells of the arteries and veins.”
One of the more surprising findings was that lymphatic cells generated in the second heart field mix with lymphatic cells with a different, likely venous, origin; the two populations together form the lymphatic vessels in the ventral part of the heart.
This result indicates that the newly discovered cell population not only contributes a large proportion of the cells of the coronary lymphatic system, but also leads a specific and irreplaceable process in the formation of the coronary lymphatic vasculature. “This function reveals, for the first time, the specialization of endothelial subpopulations in the formation of the coronary vasculature and opens the way toward a better understanding of the formation of lymphatic vessels, a process essential not only for embryonic heart development but also for the response of the heart to stress and disease in adulthood.”
Lioux, G., Liu, X., Temino, S., Oxendine, M., Ayala, E., Ortega, S., Kelly, R. G., Oliver, G., Torres, M. (2020). A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics. Developmental Cell, 52(3), 350-363. doi: 10.1016/j.devcel.2019.12.006
NATURE IMMUNOLOGY
Neutrophils are equipped with a 'disarmament' program that STOPS the immune system going 'out of control'
Scientists at the CNIC have discovered a ‘disarmament’ mechanism that protects our bodies against uncontrolled activity of the immune system. This newly identified immune control system is located in one of the most important cell types of the immune system, the neutrophil. The findings, published in Nature Immunology, could have major implications for the understanding and treatment of conditions such as myocardial infarction, stroke, and acute inflammation.
The study identifies an intrinsic cell mechanism that modifies the protein content of circulating neutrophils, leading to the progressive loss of the toxic contents of the granules and therefore a reduced capacity to form NETs. This program thus undermines the main offensive mechanism of neutrophils. The study shows that this disarmament program is driven by the receptor CXCR2 and regulators of circadian rhythms.
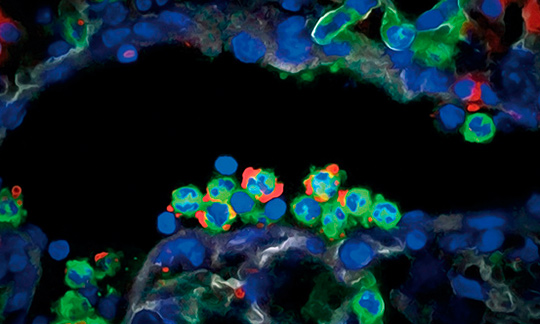
The findings show that neutrophils possess “an innate system that gradually reduces their capacity to mount a toxic attack, so that as they age, neutrophils disarm themselves before they can damage healthy tissues. There are many diseases that cause more or less damage according to the time of day, and this disarmament program helps to explain the origin of these clinical differences,” explained the researchers.
Adrover, J. M., Aroca-Crevillen, A., Crainiciuc, G., Ostos, F., Rojas-Vega, Y., Rubio-Ponce, A., Cilloniz, C., Bonzon-Kulichenko, E., Calvo, E., Rico, D., Moro, M. A., Weber, C., Lizasoain, I., Torres, A., Ruiz-Cabello, J., Vázquez, J., Hidalgo, A. (2020). Programmed 'disarming' of the neutrophil proteome reduces the magnitude of inflammation. Nature Immunology, 21(2), 135-144. doi: 10.1038/s41590-019-0571-2
NATURE COMMUNICATIONS
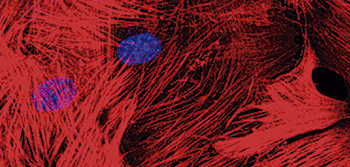
CNIC scientists design an experimental mouse model for investigating the mechanical function of proteins in vivo
The CNIC Molecular Mechanics of the Cardiovascular System group, led by Jorge Alegre Cebollada in partnership with an international scientific team, has generated the first experimental mouse model that allows direct analysis of the mechanical function of proteins in living organisms. The model, published in Nature Communications, is based on the insertion of the HaloTag-TEV module into titin, one of the proteins responsible for the elasticity of skeletal and cardiac muscle. The HaloTag-TEV module combines three key properties. Dr Alegre explained: “Thanks to the introduction of this module into the gene, we are able to fluorescently mark the protein, which makes it easy to track the module and see where it has inserted correctly.”
The module also includes a target for specific protein lysis, so that the mechanical function of the target protein can be interrupted in a controlled manner at any desired moment, allowing us to study the effect of this interruption. Finally, the module provides a way to anchor the isolated protein to surfaces, enabling the study of its mechanical properties in isolation.
“All of this helps to establish a bridge between the modulation of protein mechanical properties and observing the consequences of this modulation at a cellular level,” said Dr Alegre. The HaloTag-TEV module can be inserted into other proteins with a mechanical function, so that in the future it could be used to study other systems, including those related to diverse muscular and cardiac disorders.

Rivas-Pardo, J. A., Li, Y., Martonfalvi, Z., Tapia-Rojo, R., Unger, A., Fernández-Trasancos, A., Herrero-Galan, E., Velazquez-Carreras, D., Fernández, J. M., Linke, W. A., Alegre-Cebollada, J. (2020). A HaloTag-TEV genetic cassette for mechanical phenotyping of proteins from tissues. Nature Communications, 11(1), 2060. doi: 10.1038/s41467-020-15465-9
NATURE COMMUNICATIONS
CNIC scientists discover a new molecular mechanism that regulates the sentinel cells of the immune system
A team at the CNIC, working in partnership with researchers at Mount Sinai Hospital in New York, has discovered a new molecular mechanism mediated by nuclear receptors that determines the identity and expansion of macrophages—one of the cell types that act as immune sentinels in the body. The newly discovered mechanism specifically affects the macrophages resident in the serous cavities, the membrane-surrounded cavities that enclose and protect many organs. The findings, published today in Nature Communications, could have important implications for the treatment of diseases that affect the serous cavities and the organs they contain, including many cancers and myocardial infarction.
There are three serous membranes: the peritoneum, which covers the abdominal cavity; the pleura, surrounding the lungs; and the pericardium, which covers the heart. “One of the main functions of the macrophages residing in these cavities is to maintain homeostasis by removing dead cells,” explained study coordinator Dr Mercedes Ricote. In addition, recent studies have demonstrated that these macrophages can infiltrate adjacent injured organs, “generating an effective rapid repair response that is independent of the recruitment of macrophage precursors via the blood supply.”
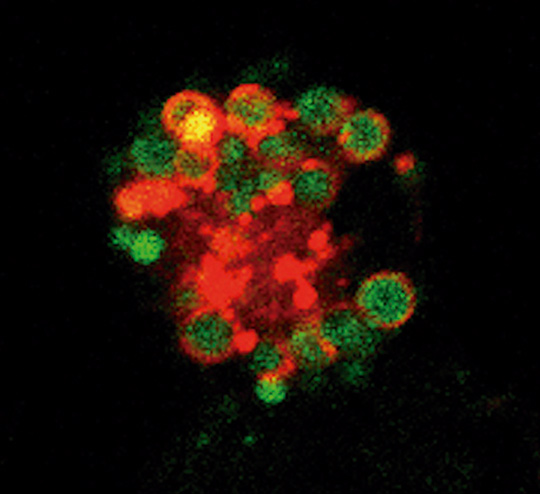
The study demonstrates that the expansion of peritoneal macrophages after birth and their maintenance during adult life are controlled by retinoid X receptor (RXR), a member of the nuclear receptor family. Using models of ovarian cancer in mice, the study shows that peritoneal macrophages can infiltrate ovarian tumors and act as ‘tumor-associated macrophages’ that “support tumor growth,” explained Dr Ricote.
The findings demonstrate that loss of RXR function leads to a decrease in the number of macrophages in the peritoneal cavity, resulting in a decreased contribution of these macrophages to growing ovarian tumors, slowing the progression of the disease. The researchers are especially interested in the possibility of modulating RXR with drugs, including some that are currently used to treat cutaneous lymphomas. “Our research could have implications for the treatment of diseases in which serous cavity macrophages contribute to disease progression, such as cancer, or to the repair of damaged tissue, as in myocardial infarction”.
The researchers are especially interested in the possibility of modulating RXR with drugs, including some that are currently used to treat cutaneous lymphomas. “Our research could have implications for the treatment of diseases in which serous cavity macrophages contribute to disease progression, such as cancer, or to the repair of damaged tissue, as in myocardial infarction”.
Casanova-Acebes, M., Menéndez-Gutierrez, M. P., Porcuna, J., Alvarez-Errico, D., Lavin, Y., García, A., Kobayashi, S., Le Berichel, J., Nunez, V., Were, F., Jiménez-Carretero, D., Sánchez-Cabo, F., Merad, M., Ricote, M. (2020). RXRs control serous macrophage neonatal expansion and identity and contribute to ovarian cancer progression. Nature Communications, 11(1), 1655. doi: 10.1038/s41467-020-15371-0
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Atherosclerosis progresses rapidly in healthy people from the age of 40
Almost half of apparently healthy people between the ages of 40 and 50 could be accumulating fatty plaques—atheromas—in their arteries at a much faster rate than was previously thought. The Journal of American College of Cardiology (JACC) has today published new data from the PESA-CNIC-Santander study demonstrating that atheroma plaques extend rapidly through the arteries of 40% of asymptomatic individuals aged between 40 and 50 years.
Researchers at the CNIC, led by Dr Valentín Fuster, Director of the CNIC and lead investigator on the PESA-CNIC-Santander study, have also found that the progression of atherosclerosis is directly related to classical cardiovascular risk factors: age, sex, hypertension, cholesterol, smoking, and diabetes. The researchers conclude that the findings, although they await validation from future events in the PESA cohort, will be of great value for identifying strategies to stall the cardiovascular disease epidemic.
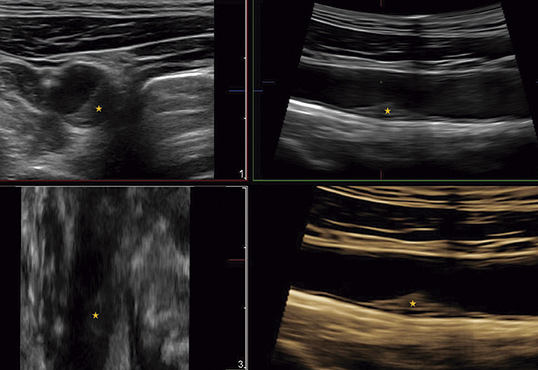
López-Melgar, B., Fernández-Friera, L., Oliva, B., García-Ruiz, J. M., Sánchez-Cabo, F., Bueno, H., Mendiguren, J. M., Lara-Pezzi, E., Andrés, V., Ibanez, B., Fernández-Ortiz, A., Sanz, J., Fuster, V. (2020). Short-Term Progression of Multiterritorial Subclinical Atherosclerosis. Journal of the American College of Cardiology, 75(14), 1617-1627. doi: 10.1016/j.jacc.2020.02.026
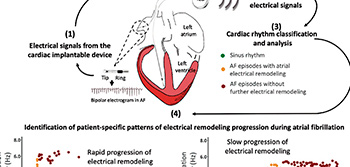
EUROPACE
Personalized medicine for atrial fibrillation
Patients with atrial fibrillation, the most frequent cardiac arrhythmia, are closer to accessing personalized medicine. This is the claim of a new study led by Dr David Filgueiras, of the CNIC, the Hospital Clínico San Carlos de Madrid, and the Centro de Investigación en Red de Enfermedades Cardiovasculares (CIBERCV). The study results show that it is possible to monitor and predict individual progression of atrial fibrillation from cardiac electrical signals obtained from implantable devices (pacemakers or defibrillators).
The study, published in the latest edition of Europace, involved the participation of 51 Spanish hospitals and the Fundación Interhospitalaria para la Investigación Cardiovascular. The results show that cardiac electrical signals from patients fitted with pacemakers or implantable cardioverter–defibrillators can be used to monitor and predict the progression of the arrhythmia in a personalized and specific manner. This is achieved with standard data transmission technology installed in implantable devices such as pacemakers. This technology can be used to monitor cardiac electrical activity during episodes of atrial fibrillation, thus establishing disease status and the rate of progression.

According to Dr Filgueiras, “this technology opens up enormous possibilities in personalized medicine for atrial fibrillation patients because it allows us to determine the progression rate of the arrhythmia in each individual and to optimize the timing of medical intervention with current treatment options.”
In addition, Dr Julián Villacastín, director of the Instituto Cardiovascular del Hospital Clínico San Carlos and an author on the study, this new approach to atrial fibrillation diagnosis will allow physicians “to monitor the influence of different interventions on disease progression.”
Lillo-Castellano, J. M., González-Ferrer, J. J., Marina-Breysse, M., Martínez-Ferrer, J. B., Pérez-Alvarez, L., Alzueta, J., Martínez, J. G., Rodríguez, A., Rodríguez-Pérez, J. C., Anguera, I., Vinolas, X., García-Alberola, A., Quintanilla, J. G., Alfonso-Almazan, J. M., García, J., Borrego, L., Canadas-Godoy, V., Pérez-Castellano, N., Pérez-Villacastin, J., Jiménez-Diaz, J., Jalife, J., Filgueiras-Rama, D. (2020). Personalized monitoring of electrical remodelling during atrial fibrillation progression via remote transmissions from implantable devices. Europace, 22(5), 704-715. doi: 10.1093/europace/euz331
SCIENCE ADVANCES
THE MECHANISM THAT REGULATES MITOCHONDRIAL ENERGY PRODUCTION
CNIC scientists have identified the molecular mechanism by which mitochondria—the main source of the cell's energy supply—regulate their function to optimize energy production in order to meet the body’s changing needs. The discovery, published in the journal Science Advances, helps to explain how the body regulates its metabolism.
The study, led by CNIC scientists Jesús Vázquez and José Antonio Enríquez, shows that mitochondria adjust the efficiency of the electron transport chain (ETC) to meet the body’s needs by regulating the associations between its component macromolecular structures. The researchers found that the protein SCAF1, discovered by the same team in 2016, plays a key role in this metabolic regulation by optimizing mitochondrial energy efficiency in response to high energy demand.

The research team conclude that SCAF1-mediated physical interaction between CIII and CIV is essential for optimal mitochondrial energy production. SCAF1 is a regulatory factor that allows mitochondria to adapt to the available nutrient sources of sugars, fats, or proteins. This capacity for metabolic adaptation also explains the ability of mitochondria to adapt to stress situations, for example during intense physical exercise.
The study was supported by funding from The International Human Frontier Science Program Organization (HFSP RGP0016/2018), Fundació Marató.TV3, and the “la Caixa” Foundation.
Calvo, E., Cogliati, S., Hernansanz-Agustin, P., Loureiro-López, M., Guaras, A., Casuso, R. A., García-Marques, F., Acin-Pérez, R., Marti-Mateos, Y., Silla-Castro, J. C., Carro-Alvarellos, M., Huertas, J. R., Vázquez, J., Enriquez, J. A. (2020). Functional role of respiratory supercomplexes in mice: SCAF1 relevance and segmentation of the Qpool. Science Advances, 6(26), eaba7509. doi: 10.1126/sciadv.aba7509
SCIENCE ADVANCES
Spanish scientists discover a system essential for limb formation during embryonic development
Researchers at the CNIC have discovered a system that provides cells with information about their position within developing organs. This system, studied in developing limbs, tells cells what anatomical structure they need to form within the organ. The article, published in Science Advances, shows that malfunctioning of this system causes congenital malformations and could in part explain the effect of thalidomide, a drug contraindicated in pregnancy because it induces limb defects.
The CNIC team, working with partners at the National Institutes of Health in the USA, analyzed the molecular basis of limb formation. The scientists discovered how cells obtain information about their position on the proximodistal axis of the limb bud, or primordium (the rudimentary state of a developing organ.)
The study shows that the signal that tells cells where they are is the growth factor FGF. The strength of the signal received by cells depends on how close they are to the FGF-producing cells at the distal tip of the limb bud.
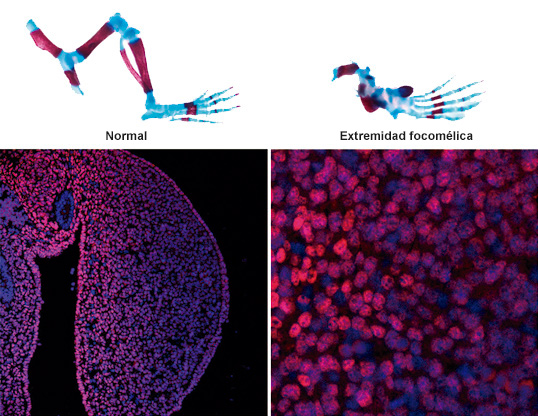
The researchers demonstrated that the molecule in receptor cells that interprets FGF signals is a transcription factor called Meis. This transcription factor is distributed in a linear abundance gradient, so that it is highly abundant in proximal cells (close to the body trunk) and becomes progressively less abundant in more distal positions. This system, emphasized Torres, “is essential for the correct formation of the limbs.” The mechanisms described in the study advance understanding of the origin of phocomelia, a congenital defect in which the embryonic limbs only form hands and feet, with the rest of the limb failing to develop. In the study, experimental elimination of FGF–Meis signaling resulted in all limb-bud cells receiving the incorrect instruction that they were distal, resulting in phocomelia.
The findings establish a new model for the generation of proximodistal identity in the developing vertebrate limb and provide a molecular mechanism for the interpretation of FGF gradients during axial patterning in vertebrate embryos.
Delgado, I., López-Delgado, A. C., Rosello-Diez, A., Giovinazzo, G., Cadenas, V., Fernández-de-Manuel, L., Sánchez-Cabo, F., Anderson, M. J., Lewandoski, M., Torres, M. (2020). Proximo-distal positional information encoded by an Fgf-regulated gradient of homeodomain transcription factors in the vertebrate limb. Science Advances, 6(23), eaaz0742. doi: 10.1126/sciadv.aaz0742
SCIENCE ADVANCES
CNIC scientists discover the mechanism of competition between mitochondrial genomes coexisting in the same cell
Research at the CNIC has identified the mechanism of competition between distinct mitochondrial genomes coexisting in the same cell. The study, published today in Science Advances, examines why the simultaneous presence of more than one variant of mitochondrial DNA (mtDNA) in a cell is rejected in most tissues, which select a single mtDNA variant that differs between different tissues.
The results show that cells are able to detect and select between mitochondria with distinct mtDNA variants that make them more or less efficient for different cell types depending on their metabolic needs. This explains how the preferred mtDNA variant can differ between cell types.
The study establishes that the selection of a mtDNA variant depends on the cell type and not the tissue, as was thought previously.

Moreover, the researchers add, the study has identified molecular targets for the development of tools to control mtDNA selection and cell metabolism in order to prevent the accidental generation of heteroplasmy through new medical procedures. These new technologies include the transplantation of healthy mitochondria to prevent mitochondrial diseases, injection of mitochondria into oocytes to increase fertility, and the proposed transfer of mitochondria in cell therapies to treat diverse diseases, including cardiovascular, lung, and neurological diseases.
The study was supported with funding from The International Human Frontier Science Program Organization (HFSP RGP0016/2018).
Lechuga-Vieco, A. V., Latorre-Pellicer, A., Johnston, I. G., Prota, G., Gileadi, U., Justo-Mendez, R., Acin-Pérez, R., Martínez-de-Mena, R., Fernández-Toro, J. M., Jiménez-Blasco, D., Mora, A., Nicolás-Ávila, J. A., Santiago, D. J., Priori, S. G., Bolanos, J. P., Sabio, G., Criado, L. M., Ruiz-Cabello, J., Cerundolo, V., Jones, N. S., Enriquez, J. A. (2020). Cell identity and nucleo-mitochondrial genetic context modulate OXPHOS performance and determine somatic heteroplasmy dynamics. Science Advances, 6(31), eaba5345. doi: 10.1126/sciadv.aba5345
PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
A new mechanism controlling liver cancer development
CNIC researchers have discovered a mechanism controlling the development of a type of liver cancer. This study, published in The Proceedings of the National Academy of Sciences (PNAS) and partly funded by the Spanish Association Against Cancer, has identified a protein that, when blocked, dramatically reduces the impact and progression of this type of cancer, called cholangiocarcinoma. This work was possible because CNIC researchers developed an animal model in which alterations in the production of bile acids cause this type of tumor.
In the study, led by Guadalupe Sabio and Alfonso Mora, mice were bred whose livers do not contain the proteins JNK1 and JNK2. The researchers found that these two proteins control the production in the liver of bile acids, which are essential for proper fat digestion and the absorption of fat-soluble vitamins (A, D, E and K). "A lack of JNK1 and JNK2 in the liver leads to changes in the enzymes responsible for metabolizing cholesterol and bile acids," said Dr Mora. In the analyzed mice, “we observed excess blood levels of bile acids."
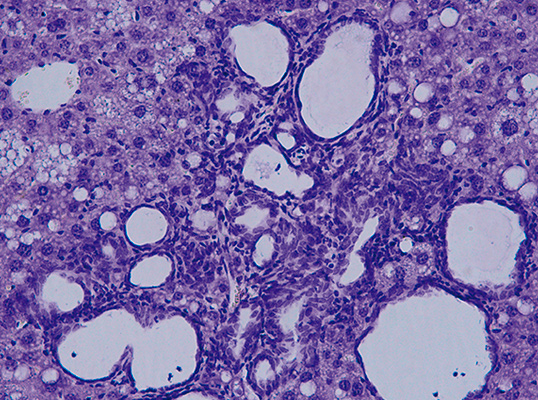
This model allowed the CNIC team to show that a key role in this tumor process is played by the protein PPARα, which regulates the metabolism of bile acids and liver lipids. According to Dr Mora, mice lacking PPARα “have significantly fewer tumors or none at all." Although it is still unknown if these data can be extrapolated to human patients, the availability of this first animal model will allow the study of a type of tumor that can still only be diagnosed in its very late stages, when metastases have already occurred.
Earlier studies had shown that JNK blockade prevented the development of steatosis in the liver, leading to a variety of clinical trials with inhibitors of these proteins.
The researchers believe that the new findings are a ‘wake-up call’ for these drugs
The study was supported by funding from the Asociación Española Contra el Cáncer and a BBVA Beca Leonardo para Investigadores y Creadores Culturales (2017).
Manieri, E., Folgueira, C., Rodríguez, M. E., Leiva-Vega, L., Esteban-Lafuente, L., Chen, C., Cubero, F. J., Barrett, T., Cavanagh-Kyros, J., Seruggia, D., Rosell, A., Sánchez-Cabo, F., Gómez, M. J., Monte, M. J., JJ, G. M., Davis, R. J., Mora, A., Sabio, G. (2020). JNK-mediated disruption of bile acid homeostasis promotes intrahepatic cholangiocarcinoma. Proceedings of the National Academy of Sciences of the United States of America, 117(28), 16492-16499. doi: 10.1073/pnas.2002672117
NATURE METABOLISM
How immune cells regulate obesity
Two CNIC groups have described how macrophages regulate obesity in a study published in Nature Metabolism that could lead to the design of treatments for obesity and overweight, as well as for related conditions such as fatty liver disease and type 2 diabetes.
The study, led by Drs José Antonio Enríquez and David Sancho, explains how fatty tissue inflammation and obesity emerge from the activation of mitochondrial metabolism in macrophages in response to oxidative stress due to excess nutrients.
The research analyzed how macrophage metabolic changes regulate the inflammatory process underlying obesity and metabolic syndrome. The new findings reveal how the detection by macrophages of oxidative danger signals—known as reactive oxygen species—leads to mitochondrial metabolism changes in these immune cells essential for their differentiation to the proinflammatory M1 macrophage type. This oxidative stress is found in morbidly obese patients and seems to be related to the high-fat diet typical in developed economies.
A key finding of the study is that a reduction of this oxidative stress ameliorates some of the harmful parameters associated with obesity.

The results support the potential use of specific Fgr protein inhibitors to treat patients with obesity or metabolic syndrome. The goal would be to reduce the inflammation associated with these conditions, reducing the impact of associated diseases fatty liver and type 2 diabetes and contributing to increased life expectancy and quality-of-life.
The study was supported by funding from the Human Frontier Science Program Organization (HFSP RGP0016/2018).
Acin-Pérez, R., Iborra, S., Marti-Mateos, Y., Cook, E. C. L., Conde-Garrosa, R., Petcherski, A., Munoz, M. D. M., Martínez de Mena, R., Krishnan, K. C., Jimenez, C., Bolanos, J. P., Laakso, M., Lusis, A. J., Shirihai, O. S., Sancho, D., Enriquez, J. A. (2020). Fgr kinase is required for proinflammatory macrophage activation during diet-induced obesity. Nature Metabolism, 2(9), 974-988. doi: 10.1038/s42255-020-00273-8
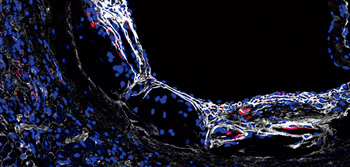
CELL
A cell-cleaning system keeps hearts healthy
Two CNIC groups, led by Drs Andrés Hidalgo and José Antonio Enríquez, have discovered a cell-cleaning system that is key to keeping a healthy heart. This mechanism allows the heart's contractile cells (the cardiomyocytes) to release damaged components outside the cell into particles called exophers. These exophers are then taken up by a network of immune cells living inside the heart, the macrophages, which are in charge of removing them before they cause inflammation in the heart.
The study, published in Cell, compiles the results of over five years of research, with collaborations with laboratories in Europe, Asia and the US. The insights that this study provides suggest that cardiac dysfunction can emerge in some instances from defects in resident immune cells, rather than from cardiomyocytes. This finding has important implications for the diagnosis and treatment of heart disease.
The fact that the heart requires a population of macrophages to do their cleaning work, among other tasks, suggests that many heart diseases of unknown origin can be explained by failure of these macrophages,” said Dr Enriquez.
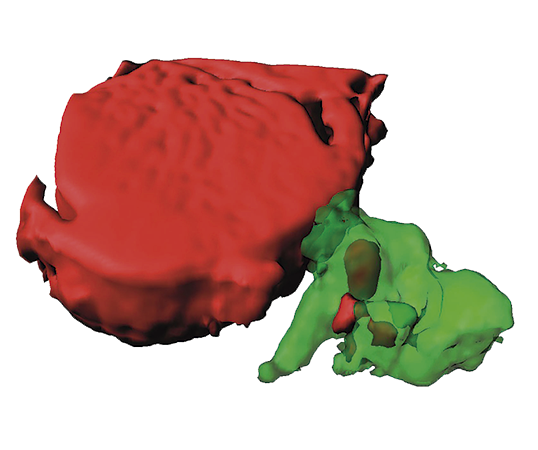
Another potential implication is that there may be similar processes supporting the fitness of specialized cells in other tissues, including the brain, whose cells share features with cardiomyocytes.
The authors conclude that the identification and elimination of cardiomyocyte-derived mitochondria and other material by resident macrophages establishes a new paradigm of how resident phagocytes contribute to general tissue maintenance.
Nicolás-Ávila, J. A., Lechuga-Vieco, A. V., Esteban-Martínez, L., Sánchez-Diaz, M., Diaz-García, E., Santiago, D. J., Rubio-Ponce, A., Li, J. L., Balachander, A., Quintana, J. A., Martínez-de-Mena, R., Castejon-Vega, B., Pun-García, A., Traves, P. G., Bonzon-Kulichenko, E., García-Marques, F., Cusso, L., N, A. G., González-Guerra, A., Roche-Molina, M., Martín-Salamanca, S., Crainiciuc, G., Guzman, G., Larrazabal, J., Herrero-Galan, E., Alegre-Cebollada, J., Lemke, G., Rothlin, C. V., Jimenez-Borreguero, L. J., Reyes, G., Castrillo, A., Desco, M., Munoz-Canoves, P., Ibanez, B., Torres, M., Ng, L. G., Priori, S. G., Bueno, H., Vázquez, J., Cordero, M. D., Bernal, J. A., Enriquez, J. A., Hidalgo, A. (2020). A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell, 183(1), 94-109. doi: 10.1016/j.cell.2020.08.031
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Additional data on the efficacy of blood thinners in COVID-19 patients
Anticoagulation therapy improves survival among hospitalized COVID-19 patients by helping to prevent fatal events associated with coronavirus, such as infarction and stroke. The study, published in The Journal of the American College of Cardiology and led by CNIC Director Dr Valentín Fuster, shows that hospitalized COVID-19 patients treated with anticoagulants—drugs that prevent blood clotting—had a better survival rate than those who did not.
At the start of the pandemic, the same research team demonstrated that anticoagulation therapy was associated with superior survival among hospitalized COVID-19 patients. This subsequent observational study showed that patients on both a “therapeutic” or full dose, and those on a “prophylactic” or lower dose, showed about a 50% higher chance of survival, and roughly a 30% lower chance of intubation, than those not on anticoagulants.
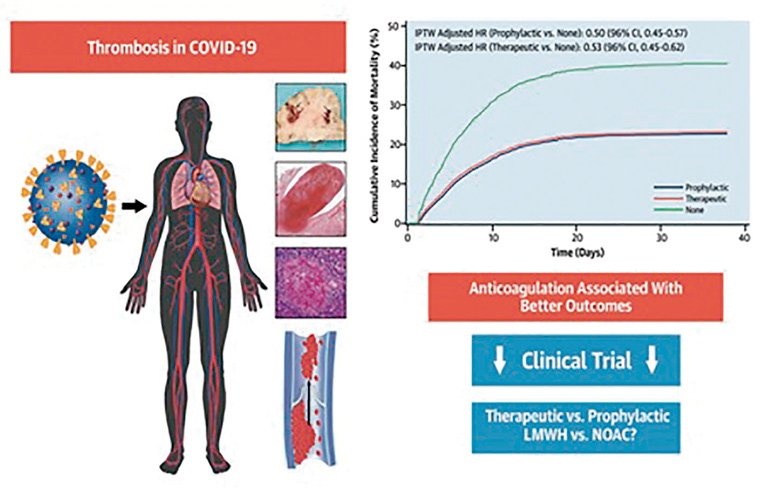
The study examined 6 anticoagulant treatment regimens. Among these, the best outcomes were obtained with a therapeutic dose.
Nadkarni, G. N., Lala, A., Bagiella, E., Chang, H. L., Moreno, P., Pujadas, E., Arvind, V., Bose, S., Charney, A. W., Chen, M. D., Cordon-Cardo, C., Dunn, A. S., Farkouh, M. E., Glicksberg, B., Kia, A., Kohli-Seth, R., Levin, M. A., Timsina, P., Zhao, S., Fayad, Z. A., Fuster, V. (2020). Anticoagulation, Mortality, Bleeding and Pathology in Patients Hospitalized with COVID-19: A Single Health System Study. Journal of the American College of Cardiology, 76(16), 1815-1826. doi: 10.1016/j.jacc.2020.08.041
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Vigorous exercise, spongy heart
Scientists at the CNIC and CIBERCV have used cardiac magnetic resonance technology to measure exercise-related hypertrabeculation in a general, non-athlete population. The results of the study have important practical implications because “misdiagnosis of noncompaction cardiomyopathy in people who exercise regularly (whether professional athletes or amateurs) can trigger medical recommendations to stop physical exercise unnecessarily,” explained CNIC General Director Valentín Fuster.
The authors conclude that cardiac magnetic resonance criteria for diagnosing noncompaction cardiomyopathy should not be interpreted in isolation. Instead, imaging results should be placed in the context of other clinical parameters, genetic tests, and the level of physical activity. This is important even in a population of non-athletes in order to avoid misdiagnosis of the disease. Misdiagnosis can result in the unnecessary cessation of exercise, with all its associated negative physical and psychological consequences.
The study forms part of the PESA-CNIC-SANTANDER study.
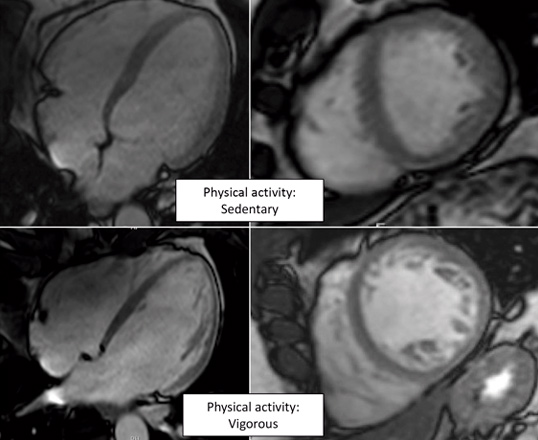
De la Chica, J. A., Gómez-Talavera, S., García-Ruiz, J. M., García-Lunar, I., Oliva, B., Fernández-Alvira, J. M., López-Melgar, B., Sánchez-González, J., de la Pompa, J. L., Mendiguren, J. M., Martínez de Vega, V., Fernández-Ortiz, A., Sanz, J., Fernández-Friera, L., Ibanez, B., Fuster, V. (2020). Association Between Left Ventricular Noncompaction and Vigorous Physical Activity. Journal of the American College of Cardiology, 76(15), 1723-1733. doi: 10.1016/j.jacc.2020.08.030
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
A new algorithm for personalized cardiovascular risk estimation in healthy people
CNIC scientists have designed an algorithm that provides a personalized estimate of cardiovascular risk in healthy middle-aged individuals based on a range of variables including age, blood pressure, diet, and blood and urine markers. The EN-PESA algorithm is an affordable tool for estimating the severity of subclinical atherosclerosis—characterized by the deposition of fatty substances in the arterial walls—especially in individuals at higher risk. The researchers conclude that EN-PESA will “help to personalize the estimation of cardiovascular risk, leading to tailored treatments and follow-up plans.” The study is published in The Journal of American College of Cardiology.
The success of machine learning algorithms derives from the analysis and systematic processing of huge quantities of data collected from a large number of individuals. One of the pioneering studies in this area is PESA-CNIC-SANTANDER, led by Dr Valentín Fuster.
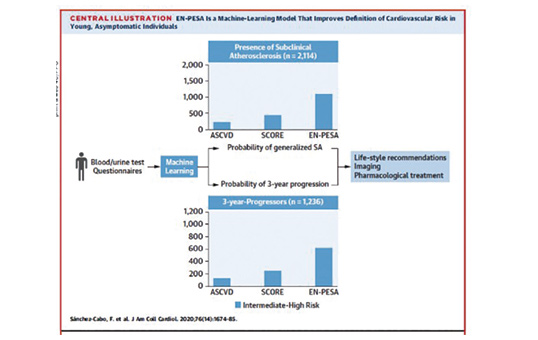
Sifting through this immense quantity of information, the EN-PESA algorithm identified a small set of variables that are easily measured in primary care centers. These variables accurately predict the extent of subclinical atherosclerosis and disease progression in middle-aged individuals who are classed at low or intermediate risk according to established cardiovascular risk scales.
The parameters include age, blood pressure, data collected in routine blood and urine analysis, and answers to dietary questionnaires.
The authors concluded that “this algorithm will improve the clinical management of apparently healthy individuals with a low cardiovascular risk according to established risk scores but who have a generalized extent of subclinical atherosclerosis or a high short-term risk of significant disease progression.”
Sánchez-Cabo, F., Rossello, X., Fuster, V., Benito, F., Manzano, J. P., Silla, J. C., Fernández-Alvira, J. M., Oliva, B., Fernández-Friera, L., López-Melgar, B., Mendiguren, J. M., Sanz, J., Ordovás, J. M., Andrés, V., Fernández-Ortiz, A., Bueno, H., Ibáñez, B., García-Ruiz, J. M., Lara-Pezzi, E. (2020). Machine Learning Improves Cardiovascular Risk Definition for Young, Asymptomatic Individuals.
SCIENCE
A new cellular defense mechanism against viral and bacterial infection
A study published in the journal Science and coordinated by researchers at the CNIC, IDIBAPS, and the University of Barcelona describes a hitherto unknown immune defense mechanism orchestrated by lipid droplets (LDs), cellular organelles able to attract and eliminate invading pathogens. LDs accumulate fatty nutrients that provide the energy cells need to perform their functions. For example, LDs provide the energy for the heart to beat, the liver to perform its metabolic functions, and muscles to move.
The newly identified mechanism highlights the intimate relationship between metabolism and immunity, and could stimulate the development of new therapeutic strategies at an especially critical time, when antibiotic resistance is presenting an ever increasing threat to public health.
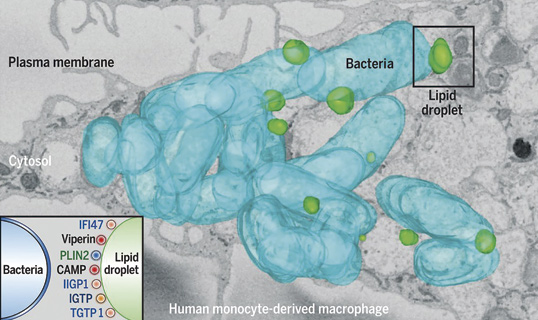
This study represents a paradigm shift because until now LDs were thought to be at the service of viruses or bacteria during infection. "In light of the widespread resistance to current antibiotics, this study has deciphered an important defense mechanism that could stimulate the development of new therapeutic strategies to stop infections.”
The study was supported by funding from the Human Frontier Science Program Organization.
Bosch, M., Sánchez-Alvarez, M., Fajardo, A., Kapetanovic, R., Steiner, B., Dutra, F., Moreira, L., López, J. A., Campo, R., Mari, M., Morales-Paytuvi, F., Tort, O., Gubern, A., Templin, R. M., Curson, J. E. B., Martel, N., Catala, C., Lozano, F., Tebar, F., Enrich, C., Vázquez, J., Del Pozo, M. A., Sweet, M. J., Bozza, P. T., Gross, S. P., Parton, R. G., Pol, A. (2020). Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science, 370(6514), eaay8085. doi: 10.1126/science.aay8085
NATURE
A key mechanism in hypoxia
Researchers at the CNIC and the Instituto de Investigación Sanitaria del Hospital Universitario de La Princesa (IIS Princesa) have made a major advance toward deciphering the mechanism through which the production of reactive oxygen species (ROS) increases in the early phases of hypoxia (acute reduction in tissue oxygen). The finding represents an important advance in the understanding of cell physiology and could be exploited in the future to treat a variety of diseases in which hypoxia plays a role, such as stroke and heart attack. The study was published in Nature.
The study shows that sodium ions act as second messengers that regulate mitochondrial function, specifically the function of the mitochondrial electron transport chain (ETC), by inducing the controlled production of ROS.

This mechanism of ROS production is fundamental to the ability of the pulmonary circulation to respond to hypoxia by redistributing blood flow to regions with less irrigation, a phenomenon known as hypoxic pulmonary vasoconstriction.
Several of the study findings provide important information about cell physiology. First, the study shows that mitochondrial sodium regulates cell membrane fluidity. This was unknown before and could have major implications for the understanding of a multitude of cellular processes.
A second important discovery is that the mitochondrial supercomplexes of the ETC play an important role in this process by adopting structural conformations that are sensitive or insensitive to sodium, thus ensuring that the action of sodium is nontoxic.
Finally, the study shows that inhibition of the mitochondrial Ca2+/Na+ exchanger NCLX is sufficient to block this pathway, preventing hypoxia adaptation. This finding indicates that NCLX could be a future therapeutic target for diseases in which hypoxia plays a role.
The study results reveal that sodium controls OXPHOS function and cell signaling in hypoxia through an unexpected interaction with phospholipids, with major consequences for cell metabolism.
The study was supported by funding from The International Human Frontier Science Program Organization (HFSP RGP0016/2018).
Hernansanz-Agustin, P., Choya-Foces, C., Carregal-Romero, S., Ramos, E., Oliva, T., Villa-Pina, T., Moreno, L., Izquierdo-Alvarez, A., Cabrera-García, J. D., Cortes, A., Lechuga-Vieco, A. V., Jadiya, P., Navarro, E., Parada, E., Palomino-Antolin, A., Tello, D., Acin-Pérez, R., Rodríguez-Aguilera, J. C., Navas, P., Cogolludo, A., López-Montero, I., Martínez-Del-Pozo, A., Egea, J., López, M. G., Elrod, J. W., Ruiz-Cabello, J., Bogdanova, A., Enriquez, J. A., Martínez-Ruiz, A. (2020). Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature, 586(7828), 287-291. doi: 10.1038/s41586-020-2551-y
CELL
The unexpected repair function of neutrophils
The CNIC team lead by Dr Andrés Hidalgo has discovered that neutrophils, the most abundant cells of the innate immune system, have many more functions in the body than previously thought. This finding suggests possible new treatments for many diseases, including cancer.
In a study published in the journal Cell, the research team demonstrate that neutrophils acquire new characteristics when they arrive in a tissue and that these specialized functions help to maintain organ health.
Every day, the marrow inside our bones produces immense quantities of neutrophils. These cells then enter the bloodstream and are distributed to almost all tissues of the body. Neutrophils have a short lifespan, living for less than 24 hours. For this reason, scientists believed that these cells had a very limited capacity to adapt to their environment and adopt new functions.
But the authors of the Cell study found that when neutrophils leave the circulation and migrate into tissues they acquire new, previously unknown properties.
According to Dr Hidalgo, the most fascinating finding is that neutrophils appear to acquire functions useful to the specific tissues in each organ.
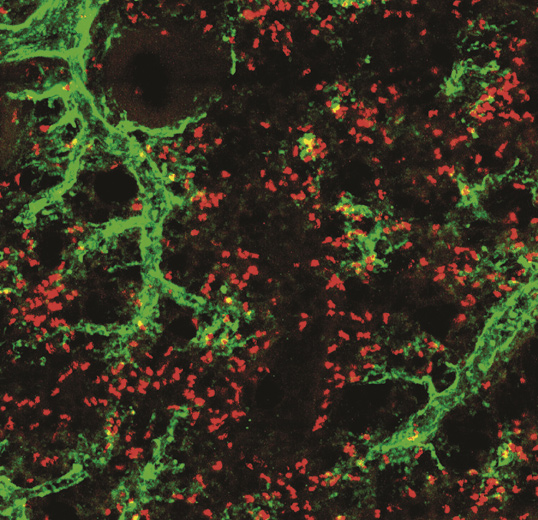
This ability to change cell properties was identified in healthy individuals, which suggests that neutrophils participate in a great variety of normal functions in the body and are not limited to combating infection. Previous studies had already identified neutrophil heterogeneity in several diseases. Indeed, these neutrophil changes are prognostic markers in cancer and help to regenerate blood cells after bone marrow transplantation.
However, the mechanisms that establish neutrophil hyperplasticity are poorly understood, and the new results are a crucial step towards filling this knowledge gap. Essentially, what the study demonstrates is that neutrophils, despite their sort lifespan, can change their function and that they do this when they enter tissues. The identification of these adaptations allows a better understanding of the roles of different immune cells in disease.
The study was supported by funding from the European Regional Development Fund (ERDF), RTI2018-095497-B-I00, the “la Caixa” Foundation, and Leducq Foundation Transatlantic Network of Excellence (TNE-18CVD04).
Ballesteros, I., Rubio-Ponce, A., Genua, M., Lusito, E., Kwok, I., Fernández-Calvo, G., Khoyratty, T. E., van Grinsven, E., González-Hernández, S., Nicolás-Ávila, J. A., Vicanolo, T., Maccataio, A., Benguria, A., Li, J. L., Adrover, J. M., Aroca-Crevillen, A., Quintana, J. A., Martín-Salamanca, S., Mayo, F., Ascher, S., Barbiera, G., Soehnlein, O., Gunzer, M., Ginhoux, F., Sánchez-Cabo, F., Nistal-Villan, E., Schulz, C., Dopazo, A., Reinhardt, C., Udalova, I. A., Ng, L. G., Ostuni, R., Hidalgo, A. (2020). Co-option of Neutrophil Fates by Tissue Environments. Cell, 183(5), 1282-1297. doi: 10.1016/j.cell.2020.10.003
ELIFE
Neutrophils, the hidden controllers of the liver’s internal clock
CNIC scientists have discovered a mechanism underlying the development of steatosis, or fatty liver, one of the main risk factors for liver cancer. The study, published in eLife, for the first time shows that neutrophils act as “circadian messengers” in the liver, controlling its internal clock and lipid metabolism. The study opens a window for potential therapies to treat liver diseases.
The study findings establish that neutrophils are active and crucial circadian regulators of liver metabolism. This could provide a new therapeutic target for the treatment of metabolic diseases such as steatosis, which commonly progresses to certain types of liver cancer.
The study was supported by funding from the Asociación Española Contra el Cáncer.

Crespo, M., González-Teran, B., Nikolic, I., Mora, A., Folgueira, C., Rodríguez, E., Leiva-Vega, L., Pintor-Chocano, A., Fernández-Chacon, M., Ruiz-Garrido, I., Cicuendez, B., Tomas-Loba, A., N, A. G., Caballero-Molano, A., Beiroa, D., Hernández-Cosido, L., Torres, J. L., Kennedy, N. J., Davis, R. J., Benedito, R., Marcos, M., Nogueiras, R., Hidalgo, A., Matesanz, N., Leiva, M., Sabio, G. (2020). Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. Elife, 9, e59258. doi: 10.7554/eLife.59258
SCIENCES ADVANCES
THE ROLE OF THE CTT PROTEIN IN THE CONTROL OF IMMUNE-SYNAPSE FORMATION
A new study published in Science Advances identifies the role of the cytosolic chaperone protein CCT in the reorganization of the cytoskeleton during the formation of an immune synapse.
The research teams led by Francisco Sánchez-Madrid and Noa Martín at the CNIC and the IIS Princesa and by José María Valpuesta at the CNB-CSIC have shown that CCT controls changes in the arrangement of the centrosome and mitochondria by regulating the production of the cytoskeletal components α- and β-tubulin.
This finding opens the path to the design of strategies aimed at blocking CCT for the treatment of autoimmune diseases that involve hyperactivation of lymphoid cells.
Ballesteros, I., Rubio-Ponce, A., Genua, M., Lusito, E., Kwok, I., Fernández-Calvo, G., Khoyratty, T. E., van Grinsven, E., González-Hernández, S., Nicolás-Ávila, J. A., Vicanolo, Martín-Cofreces, N. B., Chichon, F. J., Calvo, E., Torralba, D., Bustos-Moran, E., Dosil, S. G., Rojas-Gómez, A., Bonzon-Kulichenko, E., López, J. A., Oton, J., Sorrentino, A., Zabala, J. C., Vernos, I., Vázquez, J., Valpuesta, J. M., Sánchez-Madrid, F. (2020). The chaperonin CCT controls T cell receptor-driven 3D configuration of centrioles. Science Advances, 6(49), eabb7242. doi: 10.1126/sciadv.abb7242
NATURE REVIEWS ENDOCRINOLOGY
Stress kinases could be the next target in the treatment of obesity-associated diseases
CNIC investigators from the group led by Dr Guadalupe Sabio reviewed the key role played by stress kinases in adjusting metabolism to changing conditions in an article published in Nature Reviews Endocrinology.
Stress kinases are proteins in the body that are associated with obesity and metabolic alterations such as insulin resistance and diabetes. Understanding their mechanisms and routes of action could be crucial for the design of therapeutic strategies to combat the global epidemic of obesity, which affects millions of people worldwide.
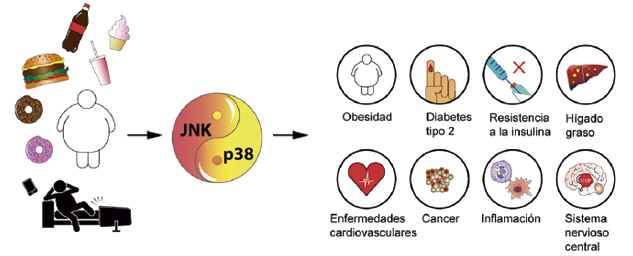
JNK and p38 SAPKs are known to act through diverse signaling pathways and mechanisms that affect metabolic processes such as insulin sensitivity, thermogenesis, and lipolysis. In addition, SAPK activity in immune cells triggers inflammation in several tissues and alters systemic metabolism in obesity. The accumulated evidence also shows that SAPKs have tissue and substrate specific functions and that their activation is involved in disorders related to obesity, steatohepatitis, liver cancer, heart failure, and diabetes mellitus.
The review concludes that the main goal in this field is to identify specific inhibitors for each of the individual p38 and JNK members. These molecules can then be tested for their ability to prevent and treat obesity and associated diseases.
Dr Sabio’s group is supported by funding from the Fundación Científica de la Asociación Española Contra el Cáncer, a BBVA Beca Leonardo para Investigadores y Creadores Culturales (2017), and the European Foundation for the Study of Diabetes.
Nikolic, I., Leiva, M., Sabio, G. (2020). The role of stress kinases in metabolic disease. Nature Reviews Endocrinology, 16(12), 697-716. doi: 10.1038/s41574-020-00418-5
CARDIOVASCULAR RESEARCH
Spanish scientists discover how the flu virus injures the heart
A study by a consortium of Spanish research groups has described the potentially fatal cardiac effects of infection with the influenza virus, especially more virulent forms. The research team showed that the replication of flu virus particles infecting the heart blocks the transmission of cardiac electrical impulses, which can lead to death. The results, published in Cardiovascular Research, highlight the potential value of testing patients with acute cardiac disease for flu infection during seasonal outbreaks of the disease. The study was carried out by scientists at the CNIC, CSIC, CIBERES, CIBERCV, and the Hospital Clínico San Carlos together with scientists at the Universities of Michigan and South Florida in the United States.
The investigators assessed the mechanisms of heart injury in mice infected with the flu virus as well as in cardiac muscle cells derived from human pluripotent cells. The results show that the most virulent viral strain persists in the heart for longer and triggers changes, including the modification of ion channels responsible for the transmission of cardiac electrical impulses. These results provide a molecular explanation for the early death of infected mice and suggest that screening for infection in acute cardiac disease patients during seasonal flu outbreaks could enable early diagnosis and treatment.
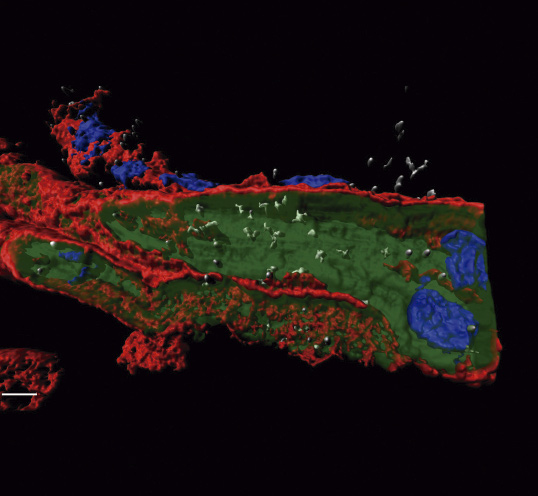
The authors concluded that “these findings highlight the potential benefit of early detection of the virus in patients with acute heart disease during flu outbreaks.”
Filgueiras-Rama, D., Vasilijevic, J., Jalife, J., Noujaim, S. N., Alfonso, J. M., Nicolás-Ávila, J. A., Gutierrez, C., Zamarreno, N., Hidalgo, A., Bernabe, A., Cop, C. P., Ponce-Balbuena, D., Guerrero-Serna, G., Calle, D., Desco, M., Ruiz-Cabello, J., Nieto, A., Falcon, A. (2020). Human Influenza A virus causes myocardial and cardiac-specific conduction system infection associated with early inflammation and premature death. Cardiovascular Research. E-published ahead of print. doi: 10.1093/cvr/cvaa117
NATURE
A new diagnostic and therapeutic target for cardiovascular disease
A study published in Nature showed that the mitochondrial protein ALDH4A1 is an autoantigen involved in atherosclerosis. The CNIC research team concluded that ADH4A1 is a potential marker for the diagnosis and treatment of cardiovascular disease (CVD).
Atherosclerosis develops for many years without causing symptoms, and there is therefore a pressing need for new tools for diagnosis and therapy. Study leader Dr Almudena Ramiro explained that, “we know that atherosclerosis includes an immunological component and that the innate and adaptive immune systems are both involved in the origin and progression of this disease.” However, little is known about the specific response of B cells in these processes or the repertoire of antibodies these cells produce during atherosclerosis.
Autoantigens are molecules produced by the body that, through a variety of mechanisms, are recognized as foreign and trigger an immune response. “ALDH4A1 is recognized by the protective antibodies produced during atherosclerosis, making it a possible therapeutic target or diagnostic marker for this disease,” commented Ramiro.
The study shows that ALDH4A1 accumulates in plaques and that its plasma concentration is elevated in the atherosclerosis-prone mice and in human patients with carotid atherosclerosis, establishing ALDH4A1 as a possible biomarker of the disease.
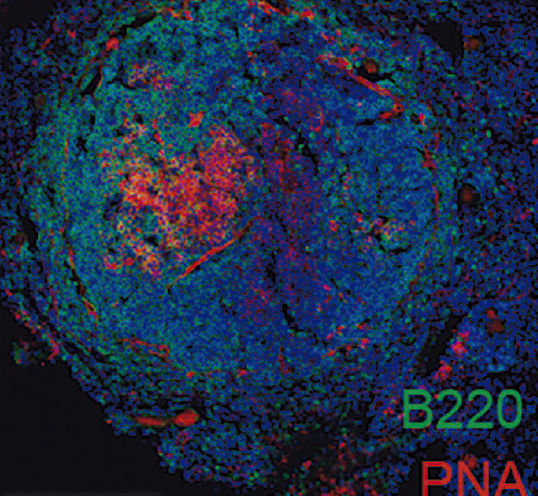
The team also found that infusion of A12 antibodies into the atherosclerosis-prone mice delayed plaque formation and reduced the circulating levels of free cholesterol and LDL, suggesting that anti-ALDH4A1 antibodies have therapeutic potential in the protection against atherosclerosis.
These results broaden knowledge of the humoral response during atherosclerosis and highlight the potential of ALDH4A1 as a new biomarker and of A12 as a therapeutic agent for this disease.
The study was supported by funding from the “la Caixa” Foundation, la Asociación Española Contra el Cáncer, and el Ministerio de Ciencia, Innovación y Universidades.
Lorenzo, C., Delgado, P., Busse, C. E., Sanz-Bravo, A., Martos-Folgado, I., Bonzon-Kulichenko, E., Ferrarini, A., González-Valdes, I. B., Mur, S. M., Roldan-Montero, R., Martínez-López, D., Martín-Ventura, J. L., Vázquez, J., Wardemann, H., Ramiro, A. R. (2020). ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature. E-published ahead of print. doi: 10.1038/s41586-020-2993-2
NATURE
A new way to make arteries
A study carried out at the CNIC not only advanced understanding of the biology of blood vessels, but also pointed the way to the design of new therapeutic strategies to induce vascularization and more effective blood perfusion of injured or ischemic tissues.
The study, led by Rui Benedito and published in Nature, reveals a new cellular and molecular mechanism essential for the development of arteries from blood capillaries, a process called arterialization. Activation of this mechanism could improve the recovery of heart function after transient or long term reduction in heart blood flow, as occurs after a heart attack.
The results also have important implications for the use of drugs to boost angiogenesis—the formation of new blood vessels—in ischemic cardiovascular disease.
The study suggests that pro-angiogenic drugs that stimulate general blood vessel proliferation will suppress arterialization. “One of our future goals will be to identify new ways to suppress proliferation signals exclusively in pre-arterial cells, thereby promoting effective arterialization without negatively interfering with the induction of capillary angiogenesis” said Dr Benedito.

From a translational standpoint, the authors conclude that the ability to modulate arterial or venous identity of blood vessels is of great interest for the treatment of coronary artery disease and myocardial infarction. The results obtained could lead to novel therapeutic approaches to induce effective arterialization in ischemic cardiovascular disease.
Luo, W., García-González, I., Fernández-Chacon, M., Casquero-García, V., Sánchez-Munoz, M. S., Muhleder, S., García-Ortega, L., Andrade, J., Potente, M., Benedito, R. (2020). Arterialization requires the timely suppression of cell growth. Nature, E-published ahead of print. doi: 10.1038/s41586-020-3018-x

Rui Benedito's Group
4. CNIC News and Views
1. A CNIC-led consortium receives funding from the la Caixa Foundation for a project to discover new therapeutic targets for atherosclerosis
The ‘la Caixa’ Foundation, as part of its 2020 Health Research Program, has awarded €999,988 to the AtheroConvergencia project, to be carried out by a consortium led by CNIC researcher Dr. Miguel Ángel del Pozo. AtheroConvergencia (Flow-driven inflammation and arterial wall remodeling in atherosclerosis: mechanisms and therapeutic potential) aims to find new markers and therapeutic targets in atherosclerosis. The project combines multidisciplinary expertise with cutting-edge technologies to gain a comprehensive understanding of atherosclerosis, identify new opportunities for precise medical intervention at advanced disease stages, and discover new genetic markers that predict the risk of developing this disease.

2. Francisco Sánchez-Madrid awarded the Santiago Ramón y Cajal National Prize
Dr. Francisco Sánchez Madrid received the Santiago Ramón y Cajal National Prize in the area of Biology. Dr Sánchez-Madrid is a group leader at the CNIC and a Full Professor in Immunology at the Universidad Autónoma de Madrid (UAM) and heads the Immunology Service at Hospital Universitario La Princesa. The committee awarded the prize to Sánchez Madrid in recognition of his contributions to biomedicine in the field of leukocyte intercellular communication, adhesion, migration, and activation and the impact his work has had on the study of chronic inflammatory diseases. These achievements, together with his high international profile throughout his scientific career, his outstanding record of training and mentoring young Spanish scientists, and the biotechnological applications of his discoveries, amply justify this award. The National Prizes for Research, created in 1982, are the highest award in Spain for scientific research.

3. Dr Valentín Fuster receives the prestigious Prize PRIze Mahidol for his scientific contributions to medicine
CNIC General Director Dr Valentín Fuster received the 29th Premio Prince Mahidol in the area of Medicine. This prestigious award from the Fundación Prince Mahidol recognizes the international leadership shown by Dr Fuster over the past 40 years through his innovative contributions to cardiovascular medicine, spanning basic research to clinical care, and more recently his tireless promotion of cardiovascular health throughout the world. The committee noted the “value of Dr Fuster’s research in unifying and extending knowledge gained through basic research to bring new benefits to patients, especially regarding antiplatelet therapy. His exceptional contribution has saved millions of lives throughout the world.” Five previous winners of this prize later went on to win the Nobel Prize.

Dr Fuster is also the first Spanish scientist with a Webometrics classification. This measure, established by the cybermetrics group at the Consejo Superior de Investigaciones Científicas (CSIC), is based on the h index, one of the most widely used tools to determine the influence of discoveries over the a scientist’s professional career. The h index was defined by the physicist Jorge Hirsch to provide a single measure of the quality of an investigator’s findings.
4. Borja Ibáñez wins the ‘Young Talent’ prize in the Premios Constantes y Vitales for biomedical research
Dr Borja Ibáñez Cabeza, Director of Clinical Research at the CNIC, was awarded the ‘Young Talent’ Prize in the 6th edition of the Premios Constantes y Vitales for biomedical research and disease prevention, an initiative of the la Sexta television channel and the Fundación AXA. These prestigious awards aim to recognize, support, and strengthen the achievements of Spanish scientists working in the areas of biomedical research and disease prevention during 2020, an especially challenging year that demonstrated the importance of this work more than ever. The prize comes with a financial award of €100,000 to support ongoing research projects.

5. DR. Guadalupe Sabio receives the ‘Young Investigator Award’ from the European Society for Clinical Investigation
The European Society for Clinical Investigation awarded CNIC scientist Guadalupe Sabio its ‘Young Investigator Award’, which recognizes the achievements of young research scientists who have made an outstanding contribution to basic, translational, or clinical biomedical research in Europe.

6. Dr Miguel Torres named an honorary member of the Latin American Academy of Sciences
The Latin American Academy of Sciences (ACAL) has named Dr Miguel Torres an honorary member in recognition of the alignment of his work with the Academy’s goals. These goals are high-quality research, scientific progress, and the integration of Latin America and the Caribbean through cooperation in research. Dr Torres and fellow honorary member Dr Ángela Nieto are the first Spanish investigators to join ACAL, whose mission is to promote and contribute to the progress of the mathematical, physical, chemical, life, and earth sciences and their use to support the human, cultural, and social integration of Latin America and the Caribbean.

7. Dr Valentín Fuster awarded the Mujerhoy ‘Premio al Compromiso Masculino’ (Men’s commitment prize) for the ‘Mujeres por el Corazón’ (Women for the Heart) campaign
Dr Valentín Fuster received the Mujerhoy ‘Premio al Compromiso Masculino’ for his health promotion work to raise awareness among women of their vulnerability to cardiovascular disease. Since 2014, Dr Fuster has led the ‘Mujeres por el corazón” campaign, a joint effort of Fundación Mapfre, the Pro-CNIC Foundation, the Community of Madrid, and the Spanish Heart Foundation. The campaign aims to raise awareness of women’s cardiovascular risk so that symptoms can be recognized early and to promote healthy lifestyle habits.
8. The SEC annual meeting holds its first sessions dedicated to basic and translational cardiovascular research
In its 2020 congress, the Spanish Society of Cardiology for the first time included sessions dedicated to basic and translational cardiovascular research. Several CNIC scientists were among the participants, including Héctor Bueno, Miguel Ángel del Pozo, Fátima Sánchez-Cabo, José Javier Fuster, Andrés Hidalgo, Miguel Torres, Guadalupe Sabio, David Filgueiras, and José Jalife.

9. CNIC named one of the 4 leading cardiovascular research centers in Europe at the 2020 European Society of Cardiology Congress
The CNIC was one of only 4 leading centers in cardiovascular research to be invited to showcase itself in a live session at the European Society of Cardiology (ESC) annual meeting, which in 2020 set a new record of more than 115,000 participants from 211 countries. The CNIC joined in the ‘Leading Cardiovascular Research Centres in Europe’ session together with Oxford University, the Stockholm Karolinska Institute, and the University Medical Center Hamburg-Eppendorf.

10. Science week 2020: A talk on Covid-19 for children and their families
As part of Semana de la Ciencia (Science Week) 2020, the CNIC organized an event for children at which Dr Miguel Ángel del Pozo spoke about the science behind SARS-CoV2 infection and the body’s immune response to it.

11. LaLiga joins the Pro-CNIC Foundation in the fight against cardiovascular disease
LaLiga and the Pro-CNIC Foundation have joined forces to promote awareness among football fans about the importance of cardiovascular disease prevention, taking care of one’s heart, and avoiding bad habits like physical inactivity and smoking, which are major causes of diseases that affect the heart. The 2 organizations have launched the Cuida tus latidos campaign (Take care of your heartbeats; www.cuidatuslatidos.com), which provides soccer fans with information on how to prevent serious cardiovascular conditions like a heart attack.

12. Visiting Researchers Program: The Jesús Serra Foundation funds the stay of Dr. Gabriel Núñez at the CNIC
The Jesús Serra Foundation, of Grupo Catalana Occidente, financed the stay at the CNIC during January-March 2020 of Dr. Gabriel Núñez, professor in the Department of Pathology at the University of Michigan (USA).
This scholarship is part of the Jesús Serra Foundation’s Visiting Researchers program and aims to promote interaction in research to contribute to scientific advances.

Promoted by the Jesús Serra Foundation with different Spanish institutions since 2009, the Visiting Researchers Program finances the stay of internationally renowned scientists so that they can visit and work in a Spanish research center over a period of several months. The goal is that the visiting researcher can, on the one hand, deepen the scientific relationship between the host research group and the researcher's center of origin, and, on the other hand, start new lines of research based on shared scientific interests. Dr. Núñez is one of the leading experts in gastrointestinal and systemic inflammation, host-microbe interactions, and mucosal immunology.
13. ERC Consolidator Grants 2020
The CNIC obtained two ERC Consolidator Grants in the 2020 call.

- “ProtMechanics-Live: Uncovering Protein Mechanics in Physiology and Disease”
- €2 million
- Jorge Alegre
- Objetive: To overcome the technical barriers to scientific progress by establishing the manipulation of protein mechanics in living cells and animals as a new field of research.

- “AngioUnrestUHD: Understanding and modulating vascular arrest with ultra-high definition”
- €2 million
- Rui Benedito
- Objetive: To use new research tools and methods to advance understanding of blood vessel iology in different physiological and pathological contexts.
Total active ERC Grants: 2 Advanced Grants, 6 ERC Consolidator Grants, and 1 Starting ERC Grant.
5. Training Programs

Training is one of the CNIC’s core activities, and the Center has devised a comprehensive training plan, the CNIC-Joven Training Plan. This global plan includes programs for participants at all levels, from high-school students to postdoctoral researchers and MDs. The CNIC-Joven Training Plan aims to fulfill the personal goal of Valentín Fuster “to attract and train the brightest young people from the earliest ages to create a pool of researchers of excellence in the field of cardiovascular research.”
PROGRAMS FOR UNDERGRADUATE STUDENTS
Curricular and Extracurricular University Practical Program
The CNIC offers practical training in cardiovascular research to visiting undergraduate students.
The CNIC has signed educational collaborative agreements with 33 Spanish Universities and 25 European Universities.
Sixteen students from the following universities worked on their final degree thesis dissertation (TFG) under the guidance of a CNIC supervisor:
- 10 students from the Autonomous University of Madrid;
- 2 students from the University Alcalá de Henares;
- 2 students from the University Politécnica of Madrid;
- 1 student from the University Francisco de Vitoria of Madrid;
- 1 student from the University Rey Juan Carlos of Madrid.
Seven students from the following universities completed an extracurricular internship at the Center under the guidance of a CNIC supervisor:
- 4 students from the University Carlos III of Madrid;
- 3 students from the Autonomous University of Madrid.
PROGRAMS FOR MASTER’S AND GRADUATE STUDENTS
Master’s Fellowship Program (CNIC-ACCIONA) and Fundación Carolina BBVA-CNIC Master’s Fellowship Program
These grants provide funding for students studying for a master’s degree at a Spanish university to carry out their experimental project (TFM) in a CNIC laboratory.
Fellowships in 2020: 11
Other MASTER'S Student Internships
Thirty-four students from the following universities worked on their final master’s thesis dissertation (TFM) under the guidance of a CNIC supervisor:
- 6 students from the Autonomous University of Madrid;
- 9 students from the Complutense University of Madrid;
- 2 students from the University San Pablo CEU, Madrid;
- 2 students from the University Alcalá de Henares;
- 2 students from the University Politécnica of Madrid;
- 1 student from the University Carlos III of Madrid;
- 1 student from the University Pompeu Fabra, Barcelona;
- 1 student from the University Oberta of Catalonia.
Insights into Research in Cardiovascular Disease Master’s Module
This postgraduate course is run by the CNIC as part of the Universidad Autónoma de Madrid (UAM)
Molecular Biosciences Master’s Program. This optional module provides a broad overview of cardiovascular biology, including perspectives from basic, clinical, and translational research.
Attendants on this course are enrolled UAM Master’s students, CNIC predoctoral researchers, and participants of the Res@CNIC SEC Program (see below).
Predoctoral (PhD) Program
The Predoctoral Program provides a unified framework for all CNIC researchers who are working toward a doctoral degree. All predoctoral researchers are signed up to this program, irrespective of their funding source.
The aims of the program are to ensure uniform quality of predoctoral training at the CNIC and further to ensure fair and equal access of predoctoral researchers to training opportunities.
The Program schedules regular meetings between the predoctoral fellow and his or her thesis committee, composed of the thesis director, another CNIC group leader, and an external expert.
Graduate students at the CNIC awarded a PhD degree in 2020: 15
Graduate students studying for a PhD degree at the CNIC in 2020: 111
PROGRAMS FOR RESIDENT MEDICAL INTERNS
RES@CNIC Program
The Res@CNIC-SEC Program (in collaboration with the Spanish Society of Cardiology, SEC) offers resident medical interns the opportunity during the first years of their specialization period to learn about the latest techniques in cardiovascular research being used in the CNIC’s laboratories, under the guidance of a CNIC scientist. Residents participating in RES@CNIC also receive training in theoretical aspects of cardiovascular research through an expert-led taught module. The Program also seeks to create links and collaborations so that on conclusion of their MIR specialization period, these professionals will have the chance to undertake research projects in their respective Hospitals in partnership with CNIC scientists.
Participants in 2020: 17 from the following hospitals:
- Complejo Asistencia Universitario de León;
- Complejo Hospitalario de Navarra;
- Hospital Clínico San Carlos;
- Hospital General Universitario de Alicante;
- Hospital Universitari Germans Trias i Pujol;
- Hospital Universitario Central de Asturias;
- Hospital Universitario de Cabueñes (Gijón);
- Hospital Universitario Fundación Jiménez Díaz;
- Hospital Universitario Miguel Servet.
INVESMIR SEC Program
The INVESMIR SEC Program offers resident medical interns the opportunity during their specialization period to further their training through a research project in one of the CNIC’s laboratories, under the supervision of a CNIC scientist.
An important aim of the program is for participants to establish contacts and collaborations with CNIC researchers that will support them, after completion of their MIR specialization training, in pursuing their own research projects at their centers within the Spanish National Health System. In 2020 two resident cardiologist interns participated in this Program from Hospital Universitario de Getafe (Madrid) and Hospital Universitario Joan XXIII (Tarragona).





PROGRAMS FOR RESEARCHERS
Cardio Joven Program
The aim of this Program (also organized in collaboration with the SEC) is to foster high-quality translational research in the cardiovascular area in Spanish National Health System centers through training programs providing theory and practical training for one cardiologist with a research vocation.
Specific aims:
-
To create the figure of the cardiologist-researcher by providing high-quality training in clinical research methods, including statistical analysis and the latest basic research techniques used in cardiovascular biomedicine, as well as opportunities to explore any clinical area of cardiology in greater depth (sub-specialization).
The program is aimed at cardiologists who aspire to carry out advanced clinical and research work at any center within the Spanish National Health System.
- International training. The Program offers a period of training toward a Master’s Degree in Epidemiology at the London School of Hygiene and Tropical Medicine (90 ECTS)
Post MIR SEA CNIC Program
This program offers 1- or 2-year contract for research into electrophysiology or arrhythmias. This contract is available to a physician completing their resident intern specialization (MIR) in cardiology and to members of the SEC Sección de Electrofisiología y Arritmias.
PRACTICAL TRAINING FOR TECHNICAL SCHOOL STUDENTS
In the fall of 2020, this program brought eight technical school students studying “Pathological and Cyto-diagnostic Anatomy” and “Clinical and Biomedical Laboratory Science” to gain practical curricular experience in the CNIC’s laboratories over a three-month period.
Moreover, in September four students studying “Diagnostic Imaging and Nuclear Medicine” and “Clinical and Biomedical Laboratory” began their 10-month internship at the CNIC as part of the DUAL technical study program, in which the second course is devoted 100% to practical laboratory-based training.
The CNIC has signed collaborative agreements for this type of internship with 19 technical training educational centers and with both DUAL Centers in Madrid offering courses in Biomedicine: the Instituto de Educación Secundaria Moratalaz for “Clinical and Biomedical Laboratory Science” and the Instituto de Educación Secundaria San Juan de la Cruz for “Diagnostic Imaging and Nuclear Medicine”.
6. Facts and Figures
Scientific Publications

| DOCUMENTS | NUMBER | |
|---|---|---|
| Articles | 255 | 78% |
| Reviews | 36 | 11% |
| Other | 36 | 11% |
| TOTAL | 327 |
LEADERSHIP
51%
CNIC-Led
publications
OPEN ACCESS
57%

| JOURNAL RANKING | |
|---|---|
| D1 | 36% |
| Q1 | 71% |
| Q2 | 18% |
| Q3 | 4% |
| Q4 | 2% |
Publications
in Top
Journals
(IF>10)
7824%

| AFFILIATION | NUMBER | |
|---|---|---|
| CNIC | 16 | 5% |
| National Collaboration | 116 | 35% |
| International Collaboration | 66 | 20% |
| National and International COLLABORATION | 129 | 39% |
Collaboration
with Hospitals
60%
Collaboration
with Universities
87%
Competitive Funding

NEW GRANTS
| National | International |
|---|---|
| 59 Grants | 14 Grants |
| €7,707,431 | € 4,102,984 |

Technology Transfer
| 6 | Patent applications |
|---|---|
| 1 | New patent application |
| 5 | Patent licensed |
| 8 | Confidentiality Disclosure Agreements |
| 1 | New Collaboration Agreement with industry |
| 56 | Material Transfer Agreements |
HUMAN RESOURCES
Scientific Staff
415
| women | |
|---|---|
| 266 | 149 |
| MEN | |
Research Areas
342
Group Leaders
31
| women | |
|---|---|
| 23 | 8 |
| MEN | |
Technical Units
73
Cardiologists
7
Heads of Technical Units
11
| women | |
|---|---|
| 5 | 6 |
| MEN | |
42(10%)
of the Scientific Staff are from outside Spain
Visiting Scientists
241
(From institutions from outside Spain 32- 17 countries including Italy, Switzerland, The Netherlands, UK, and USA)

7. Acknowledgments
BOARD OF TRUSTEES
Pedro Francisco Duque Duque
Minister of Science and InnovationRafael Rodrigo Montero
General Secretary for Science Policy Coordination Ministry of Science and InnovationRaquel Yotti Álvarez
Director, Instituto de Salud Carlos IIIMEMBERS
Silvia Calzón Fernández
- Secretary of State for Health
- Ministry of Health
Rosa Menéndez López
- President of the Spanish National Research Council (CSIC)
- Ministry of Science and Innovation
Nuria Lera Hervás
- Director of the Department for National Affairs of the
- Presidency of the Government
Margarita Blázquez Herranz
- Deputy Director General of the Cooperative Research
- Networks and Centers,
- Instituto de Salud Carlos III
Enrique Ruiz Escudero
- Health Counselor of the Madrid Region
Carlos Macaya Miguel
- Minister of Science and Innovation Representative
- President of the Spanish Heart Foundation
ELECTED MEMBERS
Luis de Carlos Bertrán
- President of the ProCNIC Foundation
Fundación Mapfre
- Julio Domingo Souto
- General Director
Banco Santander
- Rodrigo Echenique Gordillo
- Vice President
Telefónica, S.A.
- Francisco de Bergia González
- Public Affairs Director
- Deputy: Loreto Pérez del Puerto Rodríguez Public Affairs Chair
Fundación Bancaria “la Caixa”
- Antonio Vila Bertrán
- General Director
- Deputy: Angel Font Vidal Public Affairs Chair Corporate Director of Research and Strategy
SECRETARY GENERAL
Margarita Blázquez Herranz
- Deputy Director General of the Cooperative Research o
- Networks and Centers, Instituto de Salud Carlos III
LEGAL ADVISER
Juan José Torres Fernández
- State Attorney at the Supreme Court
- Deputy: Fernando Carlos Fernández de Trocóniz Marcos
SCIENTIFIC ADVISORY BOARD
Karin R. Sipido
- Division of Experimental Cardiology
- Katholieke Universiteit Leuven, Belgium
Javier Díez
- Centro de Investigación Médica Aplicada (CIMA)
- University of Navarra, Pamplona, Spain
MEMBERS
Margaret Buckingham
- Department of Developmental Biology
- Pasteur Institute, Paris, France
Ana Mª Cuervo
- Albert Einstein College of Medicine
- New York, USA
Elisabetta Dejana
- Department of Biosciences
- University of Milan (IFOM-IEO Campus), Italy
Stefanie Dimmeler
- Institute of Cardiovascular Regeneration
- Centre for Molecular Medicine, Frankfurt, Germany
Mauro Giacca
- School of Cardiovascular Medicine & Sciences
- King’s College London, UK
Stephane Heymans
- Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), The Netherlands
- Center for Molecular and Vascular Biology, University of Leuven, Belgium
Gerd Heusch
- Institute of Pathophysiology, Center of Internal Medicine
- University of Essen Medical School
- Germany
Patrizio Lancellotti
- University Hospital of Liège, Belgium
José López-Barneo
- Faculty of Medicine
- University of Seville, Spain
Filip Swirski
- Research Institute, Massachusetts General Hospital
- Boston, USA
Derek M. Yellon
- University College London
- London, UK
PUBLIC AND PRIVATE BODIES OF COMPETITIVE GRANTS
AUSTRIAN SCIENCE FUND
BANCO SANTANDER
COMUNIDAD DE MADRID
DAIICHI SANKYO FOUNDATION OF LIFE SCIENCE
EC-EUROPEAN COMMISSION
EC-EUROPEAN RESEARCH COUNCIL
EUROPEAN COOPERATION IN THE FIELD OF SCIENTIFIC AND TECHNICAL RESEARCH
EUROPEAN FOUNDATION FOR THE STUDY OF DIABETES
EUROPEAN MOLECULAR BIOLOGY ORGANIZATION
EUROPEAN SOCIETY OF CARDIOLOGY
FEDERATION OF EUROPEAN BIOCHEMICAL SOCIETIES
FUNDACION BBVA
FUNDACION CIENTIFICA DE LA AECC
FUNDACION LA CAIXA
FUNDACION LA MARATO TV3
HUMAN FRONTIER SCIENCE PROGRAM
INSTITUTO DE SALUD CARLOS III
LASEXTA Y LA FUNDACIÓN AXA
LEDUCQ FOUNDATION
MINISTERIO DE UNIVERSIDADES
MINISTERIO DE CIENCIA E INNOVACIÓN
MYOKARDIA
PROGERIA RESEARCH FOUNDATION
SOCIEDAD ESPAÑOLA DE CARDIOLOGIA
SOCIEDAD ESPAÑOLA DE INMUNOLOGÍA
SWEDISH SOCIETY FOR MEDICAL RESEARCH
UNESCO-LÓRÉAL INTERNATIONAL
8. Figures legends and credits by Research Area
Vascular Pathophysiology
1. Confocal image of an E8.5 mouse ventricle stained for B-catenin, showing the polygonal surface of ventricular cardiomyocytes. Nuclei were labeled with DAPI (blue). (José Luis de la Pompa) - volver
2. Collagen deposition in an atherosclerotic plaque in the aortic root of a progeroid Apoe-/- LmnaG609G/G609G mouse fed a high-fat diet for 8 weeks, starting at 8 weeks of age. Red, alpha smooth muscle actin; white, collagen III; blue, Hoechst 33342. (Vicente Andrés) - volver
3. Confocal microscopy merged images of ACTA2 (red) immunostaining and DAPI-stained nuclei (blue) in a primary culture of vascular smooth muscle cells. (Juan Miguel Redondo) - volver
Myocardial Pathophysiology
1. Methodology for monitoring and predicting atrial remodeling progression in patients with atrial fibrillation. (David Filgueiras) - volver
2. Liver-infiltrating neutrophils stained with anti-Mrp14 (blue) and anti-NE (red); nuclei are stained with with Sytox Green. (Magdalena Leiva, Guadalupe Sabio) - volver
3. Cardiac macrophages in CX3CR1GFP/+ mice after myocardial infarction. Representative images of 30 μm maximum intensity projections of cardiac macrophages labeled with GFP (green), CD68 (red), and DAPI (blue) from CX3CR1GFP/+ mouse hearts isolated 3 (left) and 7 (right) days after injury. (Mercedes Ricote) - volver
Cell and Developmental Biology
1. Transmission electron microghaph (TEM) of a ransversel section of the luminal side of an aortic endothelial cell. The plasma membrane (PM) and its mechanosensing invaginations, caveolae (pseudocoloured in yellow), are highlighted. (Miguel Ángel del Pozo) - volver
2. Localization of the I/A-band region of titin in neonatal mouse cardiomyocytes using HaloTag labeling (Jorge Alegre-Cebollada, Maria Rosaria Pricolo) - volver
3. Artistic rendering of the supportive role of resident macrophages in the heart. (Andrés Hidalgo) - volver
4. Multi-color high resolution imaging of a mouse heart to identify relevant cell populations. Scale bar, 50 μm (Miguel Torres, Ghislaine Lioux, Valeria Caiolfa) - volver
The Pro CNIC Foundation brings together 12 of the most important Spanish companies and foundations: Acciona, Santander Bank, BBVA, Endesa, the Mapfre Foundation, the Mutua Madrileña Foundation, the Ramón Areces Foundation, the Repsol Foundation, Inditex, la Caixa, Prisa, and Telefónica.
This innovative public-private financing formula has allowed the CNIC to reach a very high level of excellence, as recognized in the Severo Ochoa accreditation (2012-2019) and other international awards.
© Centro Nacional de Investigaciones Cardiovasculares (F.S.P.), 2020
DL: M-6523-2021