Advanced Development in Arrhythmia Mechanisms and Therapy

The Advanced Development in Arrhythmia Mechanisms and Therapy (ADAM-T) laboratory focuses on investigating from a multidisciplinary approach, the mechanisms underlying cardiac arrhythmias that occur in highly prevalent cardiovascular diseases in the general population, as well as in specific subsets at particular risk of sudden cardiac death.
The group of scientists and their collaborators have the primary goal of providing a comprehensive understanding of the molecular and cellular mechanisms involved in electrical cardiac alterations that may lead to cardiac arrhythmia. Such an endeavor is accomplished by facilitating fluent collaborations among departments, research institutions and universities, and different levels of expertise, from students to physicians. All together make it possible to establish solid basis for identifying populations at risk of cardiac arrhythmias, as well as for studying and applying new therapies aiming to control and eliminate such a risk. All laboratory members work as a multidisciplinary team to achieve translational impact in unsolved clinical needs The laboratory has demonstrated significant contributions in the field of cardiac electrophysiology during the last 11 years. A significant number of publications support the advances in the understanding of complex cardiac arrhythmias, mapping strategies and sudden cardiac death.
Since 2016, the ADAM-T laboratory has been continuously funded by the Instituto de Salud Carlos III (ISCIII) (https://eng.isciii.es/eng.isciii.es/InformacionCiudadanos/DivulgacionCulturaCientifica/DivulgacionISCIII/Paginas/Divulgacion/Que-es-el-ISCIII.html). We have studied complex wave propagation dynamics during atrial fibrillation in a highly translational pig model of persistent and long-standing persistent atrial fibrillation. We reported clinically relevant findings on single-signal detection of rotational-footprints and leading-driver regions to identify atrial areas associated with long-term atrial fibrillation maintenance (see Figure 1 below). Our results have shown that the combination of frequency and amplitude modulations (iFM and iAM, respectively), of electrical or optical signals, enable to identify specific atrial areas as targets for atrial fibrillation ablation. These results were further validated in a pilot series of patients with symptomatic persistent atrial fibrillation episodes refractory to conventional pulmonary vein isolation. This innovative approach was developed using a fully translational strategy from 2D computational simulations and optical mapping imaging in isolated hearts, to in vivo studies in pigs and further demonstration of its clinical impact in patients.
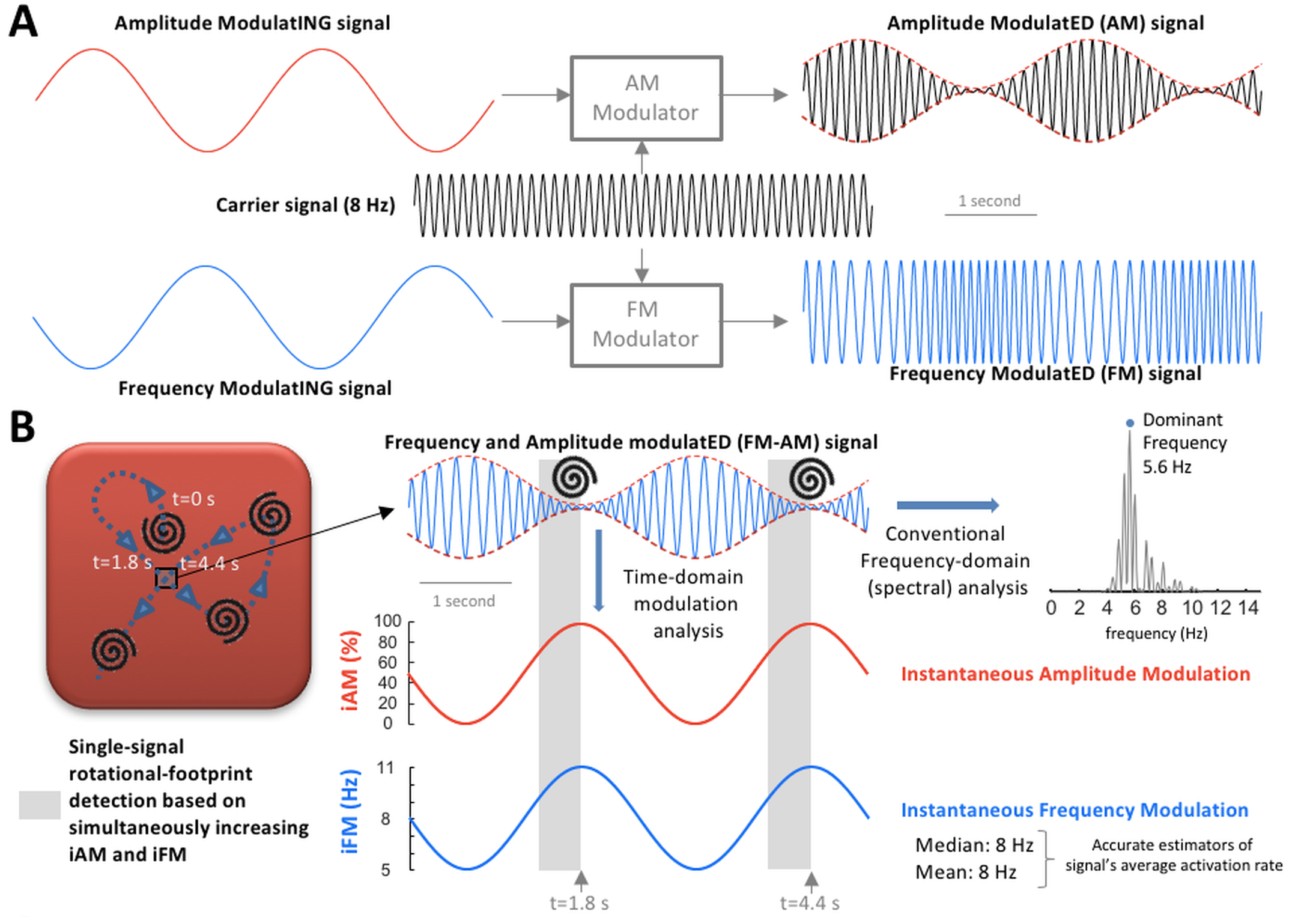
Figure 1. A and B: Amplitude modulation (AM) / frequency modulation (FM) concept. A, AM and FM in radio broadcasting. B, Left, during cardiac fibrillation, scroll wave/rotor drifting gives rise to AM and FM. When a drifting scroll wave filament/rotor core approaches the square, the amplitude of the action potential decreases increasing instantaneous AM (iAM; in red). Simultaneously, as the wave-emitting source (scroll wave filament/rotor core) approaches, the perceived instantaneous FM (iFM; in blue) at the spot increases (Doppler Effect). Therefore, simultaneous iAM/iFM increase indicates drifting scroll waves/rotors in the surroundings. Additionally, the areas with the highest average iFM are those potentially driving fibrillation in a hierarchical fashion. Right, average iFM is estimated from its median and mean values (8 Hz both) and with the conventional dominant frequency (DF) spectral approach (5.6 Hz). Interestingly, the time intervals with the highest iFM usually show the lowest amplitudes and vice versa, which conditions the height of their corresponding power spectral peaks and limits the value of DF-based hierarchical approaches. Full paper here.
This novel methodology to efficiently identify and target atrial fibrillation drivers during persistent atrial fibrillation is currently under further clinical validation in the ongoing TAILOR-AF study (NCT05169320), conducted at the Hospital Clínico San Carlos (official partner of the patent PCT/EP2019/077610). The main objective of this study is to identify (using iAM/iFM maps), target and ablate atrial fibrillation drivers (associated with atrial fibrillation maintenance) in symptomatic patients with a previous history of at least two ablation procedures. The strategy includes a catheter-based mapping and ablation procedure followed by a minimally invasive surgical approach via thoracoscopy, in cases with driver areas not suitable for catheter-based ablation. The protocol includes the study of blood biomarkers, atrial imaging and surface ECG parameters associated with atrial remodeling stages that may require a surgical approach to effectively eliminate leading driver regions. This study is a good example of the strong motivation of the ADAM-T laboratory to achieve clinical impact using novel translational tools.
To date, spatial analysis of atrial non-myocyte populations and their implications for long-term atrial fibrillation dynamics remains ill-explored. Based on the novel mapping methods to identify driver regions, we have investigated the differential regional features of non-myocyte populations in the atria of clinically relevant pig models, and humans with persistent atrial fibrillation. We have recently identified the emergence of cellular states in myeloid cells and fibroblasts associated with persistent atrial fibrillation. More importantly, we demonstrated that individual-specific regions, driving the overall arrhythmia, exhibit differential adaptive changes characterized by an enrichment in resident cardiac macrophages and a high abundance of anti-apoptotic proteins. The latter may play a critical role in cardiomyocyte homeostasis and survival within driver regions subject to long-term high activation rates.
Successful rhythm control in patients with atrial fibrillation is highly dependent on the individual-specific underlying atrial remodeling stage. Such remodeling includes structural, electrical and mechanical changes that make the arrhythmia persistent in the long term. Interestingly, these remodeling changes follow patient-specific patterns that cannot be fully characterize with regular clinical tools. During the last decade we have used remote monitoring technology to improve individual-specific characterization of atrial remodeling progression. This information can be used to establish both the rate of atrial remodeling progression and the atrial remodeling stage. Results from a multicenter study in collaboration with 51 centers in Spain have shown that cardiac electrical signals from implantable devices can be used to monitor and predict the progression of electrical remodeling progression in a patient-specific manner. Figure 2 (below) shows a schematic view of the methodology and representative results of the study. Full paper here.
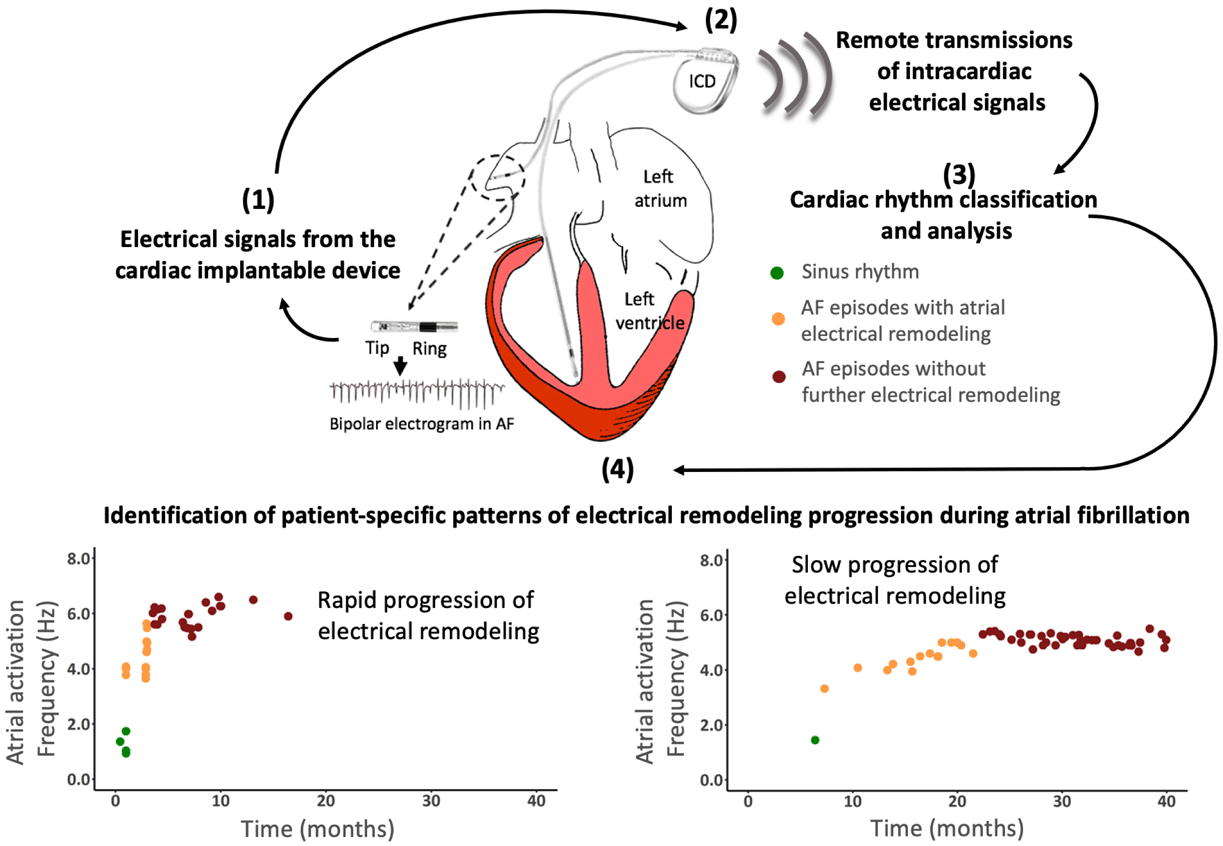
Figure 2. Medical application of remote monitoring technology to characterize patient-specific progression of atrial electrical remodeling.
Interestingly, we have documented that the pig model of persistent atrial fibrillation follows individual-specific patterns which are similar to the remodeling patterns documented in patients. These results motivated another study that reported novel mechanistic insights into the electromechanical remodeling process associated with atrial fibrillation progression and further demonstrated its prognostic value in the clinic. In pigs, long-term comprehensive monitoring of atrial fibrillation progression in vivo showed individual-specific changes in electromechanical remodeling. Advanced optical mapping techniques applied to whole heart preparations demonstrated that atrial fibrillation remodeling decreased the activation frequency threshold to observe dissociation between transmembrane voltage and intracellular free calcium signals. In patients, non-invasive assessment of the atrial electromechanical relationship demonstrated that atrial electromechanical dissociation is an early prognostic indicator for acute and long-term rhythm control. Full paper here.
Cardiac fibrillation, and more specifically, atrial fibrillation is a complex arrhythmia that requires software and imaging tools to understand the dynamics and wave-propagation patterns that can be targeted to terminate the arrhythmia. A simplified schema of the complexity of atrial fibrillation dynamics is shown in Figure 3 (below). More information can be found in Jorge G. Quintanilla et al. Cardiovasc. Res. 2021;117:1662-1681.
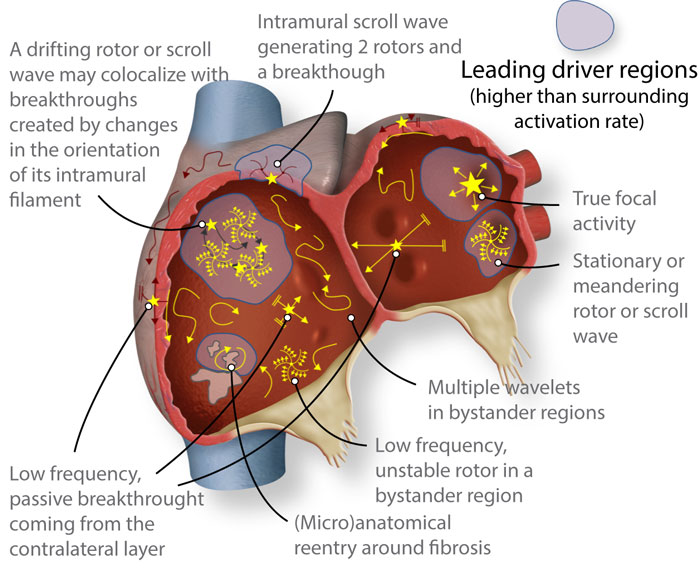
Figure 3. Schematic of structurally and electrically remodeled atria, where several reported patterns might coexist. However, atrial fibrillation may still be driven in a hierarchical fashion by specific regions with higher than surrounding activation rate (leading drivers). Such regions would host reentrant drivers in many cases, albeit not all reentrant activity would act as a driver, since rotors are commonly found in passive bystander regions.
The highly translational value of the pig model, and current gaps in knowledge to characterize atrial remodeling progression and atrial myopathy using novel biomarkers, have led to additional funding from the European Commission, under the H2020 program and the Machine Learning Artificial Intelligence Early Detection Stroke Atrial Fibrillation (MAESTRIA) consortium. The MAESTRIA project focuses on developing novel approaches for timely detection of atrial myopathy to improve care management and identifying novel therapeutic targets for personalized medicine of atrial fibrillation and stroke. More recently, the Ministerio de Ciencia e Innovación have also funded a collaborative project aiming to integrate genetic and patient-specific parameters to predict atrial fibrillation progression and the therapeutic response in a patient-specific manner.
Overall, atrial fibrillation is a long-term line of research in the lab since 2014. The laboratory will continue to take advantage of a translational pig model of long-lasting persistent atrial fibrillation closely resembling atrial fibrillation pathophysiology in the clinic. We will continue working on the general hypothesis that atrial fibrillation is a deterministic process that is sustained by atrial regions with specific electrophysiological and structural properties that enable long-term maintenance of the arrhythmia. We will also work in novel areas to understand atrial fibrillation pathophysiology, which may have clinical implications for rhythm control. In the near future, we will further investigate the role of the innate immunity and non-myocyte populations in atrial fibrillation maintenance. Two new international projects will also support new studies on antiarrhythmic drug strategies during the next 4 years.
Translational animal models and research areas are complemented with research activities in the clinic. The laboratory is carrying out clinical trials related to atrial fibrillation (https://clinicaltrials.gov/ct2/show/NCT03005366?term=Filgueiras-Rama&rank=2) and ventricular fibrillation (https://clinicaltrials.gov/ct2/show/NCT03248557?term=Filgueiras-Rama&rank=1). The laboratory also works on big data analysis from a multicenter registry of cardiac implantable devices to identify electrical parameters associated with severe ventricular arrhythmia in patients with implantable cardioverter defibrillators. This project has been approved by the UMBRELLA scientific committee, which represents a multicenter Spanish observational registry (https://clinicaltrials.gov/ct2/show/NCT01561144?term=UMBRELLA&rank=3), ensuring the legal, normative, and scientific data exploitation. The results are under validation in a multicenter study involving 8 participant centers in Spain to demonstrate the capability of advanced models to predict severe ventricular arrhythmia in patients implanted with defibrillator devices.






