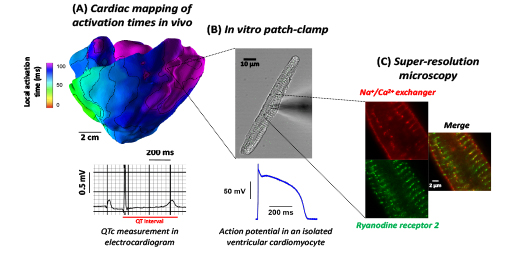
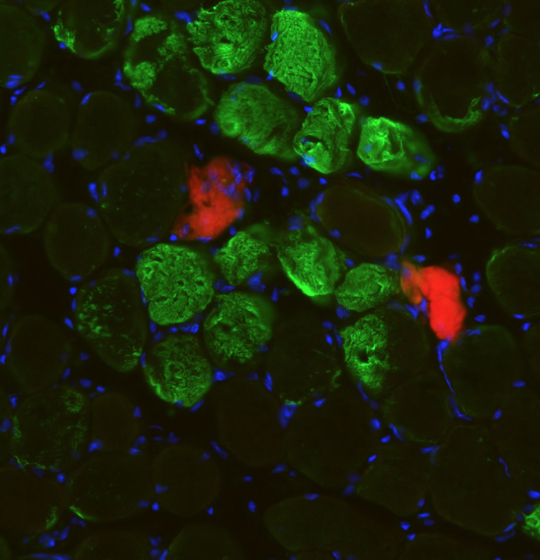
2.2 Myocardial Pathophysiology
Guadalupe Sabio
Coordinator
The Myocardial Pathophysiology Area (MPA) includes 10 research groups and 5 core technical units. MPA groups work on a wide range of topics: inherited cardiomyopathies, arrhythmia mechanisms and therapy, molecular regulation of heart failure, metabolism and its effect on cardiovascular disease, functional genetics of the oxidative phosphorylation system, translational cardiovascular imaging and therapy, molecular cardiology, immunobiology, cardiovascular health and imaging, and nuclear receptor signaling. Research in these areas produced several scientific advances during 2021.
José Antonio Enríquez research centers on the mammalian mitochondrial electron transport chain (MtETC) and H+-ATP synthase, which together comprise the oxidative phosphorylation (OxPhos) system. One key research focus is the genetic variability of mtDNA and the repercussions this has on whole-body metabolism, the response to drugs, predisposition to disease, healthy aging, and the borderline pathology and functional variability of mtDNA alterations. The group’s work has highlighted the role of mitochondrial reactive oxygen species (ROS) in OxPhos-system adaptation to the metabolic requirements of the cell. The group has also contributed to the identification of mitochondrial Na+ as a second messenger regulating inner mitochondrial membrane fluidity and ROS production by MtETC complex III. Another key research area is the role of OxPhos in metabolic adaptation, and the group has produced a key advance in the understanding of how cells optimize and regulate their metabolic capacity by inducing structural changes in the MtETC. Surprisingly, these adaptations are especially relevant in cardiovascular disease and the immune system.
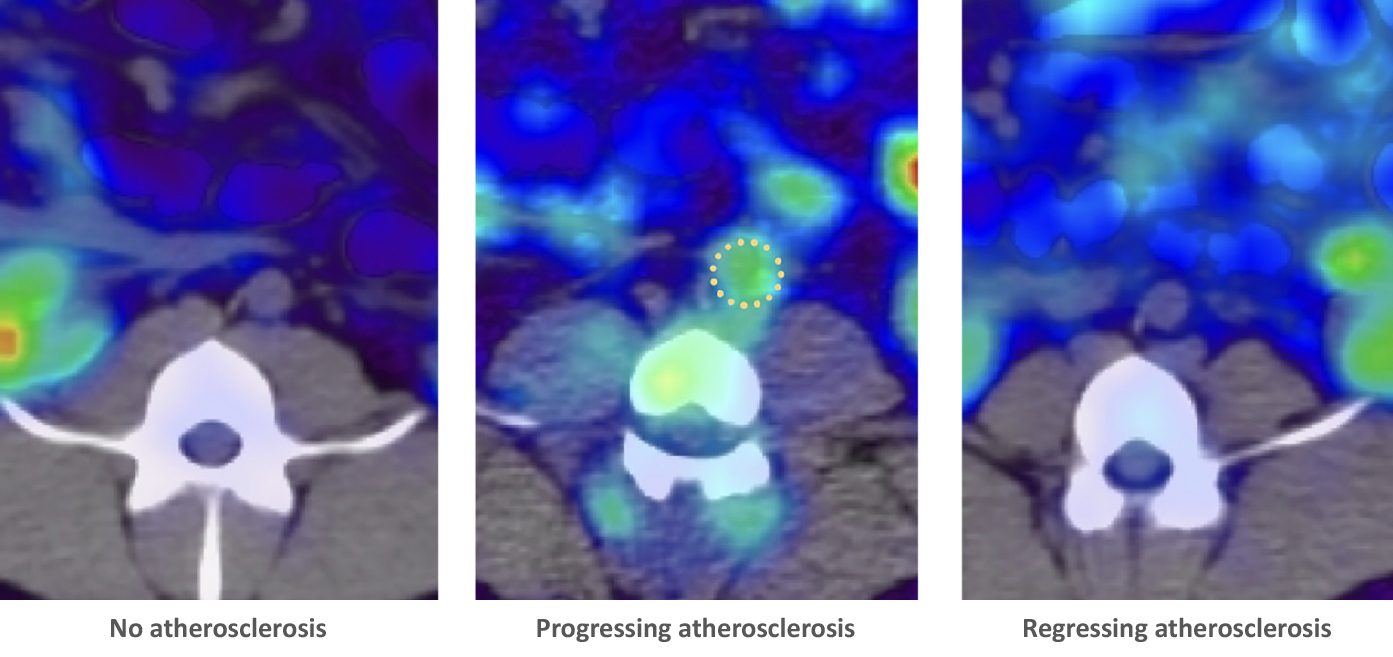
Rodrigo Fernández leads the EnIGMA (Early ImaGing Markers of unhealthy lifestyles in Adolescents) project, funded by the Fondo de Investigación Sanitaria - Instituto de Salud Carlos III. The project recruited 123 adolescents who underwent multi-territory multi-parametric CMR imaging studies at the CNIC. The first EnIGMA results will be reported soon.
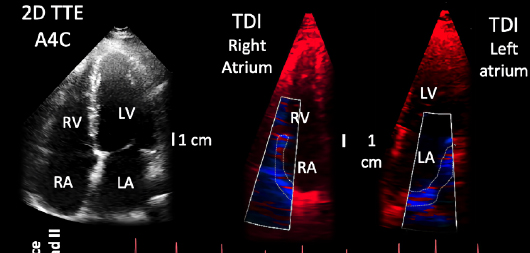
The research group led David Filgueiras has developed a novel noninvasive method to assess the relationship between mechanical and electrical activation rates during atrial fibrillation (AF), enabling the characterization of patient-specific stages of atrial remodeling as the disease progresses. The method has shown early prognostic value in rhythm control management and AF recurrences before other clinical or conventional echocardiography parameters become manifest.
The Translational Laboratory for Cardiovascular Imaging and Therapy (TLCVIT) is led by Borja Ibáñez. Continuing studies with the beta-blocker metoprolol established the use of this drug as a cardioprotective therapy against infarction and showed its utility in the treatment of critical COVID-19 patients. Research into the cardiotoxic effects of chemotherapy explored the use of remote preconditioning (RIPC) and identified microcirculation injury as an early detector of myocardial damage associated with chemotherapy drugs. The team was also central to the development of an ultrafast cardiac resonance protocol to validate a new ultrafast 3D protocol (ESSOS) for complete cardiac resonance analysis. The protocol achieves assessment of anatomy and function, as well as late enhancement to evaluate infarction, all in a single breath hold. The procedure is completed in under a minute.
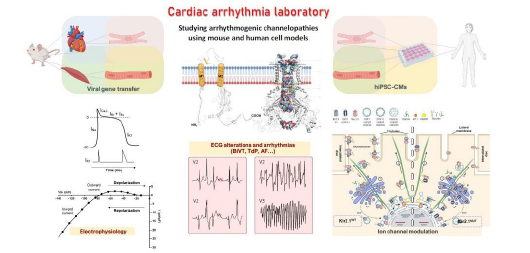
José Jalife leads the Arrhythmia Research Laboratory, which investigates the role of macromolecular ion channel complexes in the molecular mechanisms of life-threatening rhythm disturbances in patients with Andersen-Tawil syndrome, Short-QT syndrome, and Duchenne muscular dystrophy. The group adopts a multidisciplinary approach, including the use of mouse models of cardiac-specific expression of mutant potassium ion channels, patient-specific iPSC-CMs, in-silico modeling of ion channel structure-function relationships, transcriptomics, protein chemistry, patch-clamping, ECG recordings, intracardiac stimulation, and optical mapping. The ultimate goal is that these studies will identify novel targets for the prevention of arrhythmias and sudden cardiac death in patients suffering from these devastating diseases.
Enrique Lara-Pezzi leads a research group investigating the molecular mechanisms underlying the development of pathological cardiac hypertrophy. Their work shows that cardiac hypertrophy in mice is triggered by loss of the expression of SRSF4 (serine- and arginine-rich splicing factor 4), leading to diastolic dysfunction and abnormal repolarization. This response is due to the downregulation of the long noncoding RNA GAS5 (growth-arrest-specific 5) and consequent elevation of glucocorticoid receptor transcriptional activity. These findings may contribute to the development of new treatments for cardiac hypertrophy and myocardial pathology in patients with Cushing syndrome.
The CNIC Molecular Cardiology lab, led by Silvia G. Priori, aims to find new treatments for Timothy syndrome (also known as Long QT syndrome 8) and catecholaminergic polymorphic ventricular tachycardia, two highly lethal inherited diseases caused by mutations in key proteins that generate the heartbeat. Funded by Fundación La Caixa and a Plan Nacional grant, this project studies genetically modified pigs and mice that recapitulate these human diseases.
Mercedes Ricote’s lab investigates how nuclear receptors coordinate the transcriptional landscape of cardiomyocytes in homeostasis and disease. Their work has revealed that retinoid X receptors (RXRs) are essential drivers of mitochondrial fitness and nutrient balance in perinatal and adult hearts. By integrating environmental signals, these ligand-activated transcription factors promote an appropriate epigenetic remodeling that allows the expression of key genes encoding key metabolic elements, sarcomere components, and ion channels. The team’s work shows that, in addition to sustaining contractile function, RXR-controlled gene signatures are required to prevent the development of heart failure, thus establishing these nuclear receptors as therapeutic targets for treating cardiovascular disease.
Guadalupe Sabio’s lab demonstrated that p38γ/δ kinases control cardiomyocyte metabolic switching during early postnatal development. Activation of these kinases early after birth blocks glycogen production, triggering the oxidation of fatty acid by cardiomyocyte mitochondria. The teams work demonstrates that dysregulation cardiac metabolism can have whole-body metabolic consequences, showing that the heart is a key metabolic organ. These alterations in metabolic organs are due to a cardiac energetic deficit, and the studies highlights the role of heart as endocrine organ.
David Sancho’s group investigates how communication between the gut microbiota and the immune system regulates the inflammatory response, and the impact this has on cardiovascular disease. The team has found that mucosal immunotherapy with polybacterial preparations can boost innate responses through a mechanism dependent on epigenetic modifications. This effect can enhance the antiviral response to diverse viruses, including influenza A, vaccinia, and SARS-CoV-2. Trained immunity can also increase vaccine immunogenicity. However, the group is also investigating potential detrimental roles of trained immunity in atherosclerosis.
The MPA hosts five core technical units that give support to all CNIC scientists.
The Transgenesis Unit provides services in mouse strain rederivation, production of genetically modified mice, and cryopreservation of mouse strains. Through collaboration with J.N. Domínguez Macías and M. Torres, the unit is currently developing a microinjection model in post-implantation embryos.
The Pluripotent Cell Technology Unit (PCTUnit) provides state-of-the-art knowledge, training, and technological support in the culture and manipulation of mouse and human pluripotent cells. The PCTUnit has worked with CNIC researchers on the generation of several in vivo and in vitro models, including designing CRISPR/Cas9-based gene-editing strategies and performing the necessary experiments to obtain mouse and pig animal models and in vitro models of cardiovascular disease in hiPSCs.
The Clinical Trials Coordination Unit (CTCU) has continued to provide specialized support for clinical trials and studies carried out at the CNIC. Its ultimate goal is to boost Spanish leadership in clinical trials in the cardiovascular area. In 2021, the CTCU coordinated 11 clinical trials and studies that included more than 10,500 participants in more than 200 European hospitals.
The Viral Vectors Unit provides researchers with access to state-of-the-art viral vector technology for use in preclinical studies and basic research.
The Comparative Medicine Unit supports in vivo work in the animal facility.
TECHNICAL UNITS
RESEARCH GROUPS
Translational Laboratory
for Cardiovascular Imaging and Therapy
Borja Ibáñez
Stress kinases in Diabetes, Cancerand Cardiovascular Disease
Guadalupe Sabio
3. Scientific Highlights
by Publication date
Science Advances
Cnic scientists identify a mechanism through which dendritic cells improve their antiviral and immune-activation abilities
CNIC SCIENTISTS IDENTIFY A MECHANISM THROUGH WHICH DENDRITIC CELLS IMPROVE THEIR ANTIVIRAL AND IMMUNE-ACTIVATION ABILITIES
Researchers at the CNIC led by Professor Francisco Sánchez-Madrid have found that dendritic cells, which initiate specific immune responses, can reprogram their genes to improve immune response. The results of the study, published in Science Advances, could have important applications for the development of new vaccination and immunotherapy strategies.
Dendritic cells are professional antigen-presenting cells that initiate adaptive or specific immune responses. As described by the research team, “dendritic cells capture possible pathogenic agents in different tissues and entry sites, process their components, and transport them to lymph nodes. Here, they establish communication with T lymphocytes through the formation of a specialized structure called the immune synapse. The immune synapse allows the dendritic cell to present processed components of the infectious agent to a T cell, so that they can be recognized and initiate a specific T cell immune
Until now, activation of T lymphocytes was thought to be dendritic cells’ main function. However, Prof. Francisco Sánchez-Madrid’s group, working together with the group led by Dr. Almudena R Ramiro, have discovered that the dendritic cell also receives information from the T cell via the immune synapse. “The T cell sends instructions that induce a change in the dendritic cell’s gene-expression program, promoting the expression of genes related to motility, antiviral responses, and secretion and thereby increasing the dendritic cell’s capacity to generate protective anti-pathogen immune responses,” explained Sánchez-Madrid.
“This study describes how gene-expression changes are accompanied by changes in epigenetic marks on DNA. These epigenetic marks in turn produce transient changes in specific genes that promote or hinder their expression,” explained first authors Irene Fernández Delgado and Diego Calzada Fraile.
The research team found that, after participating in an immune synapse, dendritic cells migrate more efficiently to lymph nodes, where most processes involved in the activation of specific or adaptive immune responses take place.
The new study, carried out in close partnership with the CNIC Bioinformatics Unit (directed by Fátima Sánchez-Cabo) and Genomics Unit (directed by Ana Dopazo), describes a new mechanism that explains how dendritic cells improve their antiviral and immune-activation abilities.
The researchers conclude that their study shows that dendritic cells, responsible for initiating specific immune responses, reprogram their genes through altered epigenetic DNA marks after interacting with a cognate T cell. “These changes improve their motility, so that they arrive sooner at immune response activation sites, representing a new mechanism for potentiating the immune response.”
The results also have potential applications in the development of new vaccination and immune therapy strategies. For example, the described mechanism could be used to generate super-migratory post-synaptic dendritic cells able to induce stronger and more effective immune responses.
The study was supported by funding from Fundación ‘la Caixa’ through Health Research Projects call HR17-00016 and an INPhINIT ‘Retaining’ doctoral project grant”.
Alcaraz-Serna, A., Bustos-Morán, E., Fernández-Delgado, I., Calzada-Fraile, D., Torralba, D., Marina-Zárate, E., Lorenzo-Vivas, E., Vázquez, E., de Alburquerque, J. B., Ruef, N., Gómez, M. J., Sánchez-Cabo, F., Dopazo, A., Stein, J. V., Ramiro, A., & Sánchez-Madrid, F. (2021). Immune synapse instructs epigenomic and transcriptomic functional reprogramming in dendritic cells. Science Advances, 7(6), eabb9965. https://doi.org/10.1126/sciadv.abb9965
Journal of the American College of Cardiology
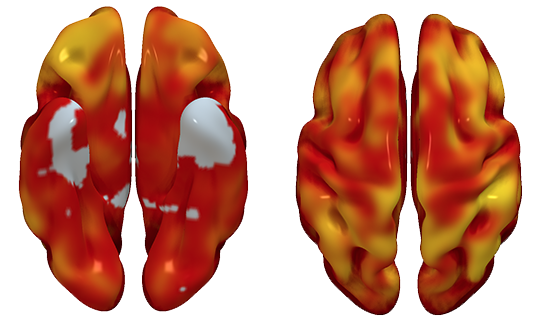
Scientists uncover early links between cardiovascular risk and brain metabolism
Scientists uncover early links between cardiovascular risk and brain metabolism
The links between cardiovascular disease and cognitive impairment begin years before the appearance of the first clinical symptoms of either condition. In a study carried out at the CNIC in partnership with Santander Bank and neuroimaging experts at the Barcelonaβeta Brain Research Center (BBRC, the research center of the Fundación Pasqual Maragall), the investigators have identified a link between brain metabolism, cardiovascular risk, and atherosclerosis during middle age, years before the first appearance of symptoms.
The report, published in the Journal of the American College of Cardiology (JACC), is important because it suggests that intervention in a modifiable condition (cardiovascular disease) could prevent the development of dementia, a disease for which there is currently no cure.
Dr. Valentín Fuster, CNIC and Mount Sinai Heart General Director, Physician-in-Chief of the Mount Sinai Hospital, and a lead author on the study, explained that “although everybody knows about the importance of caring for ourselves and controlling cardiovascular risk factors in order to avoid a heart attack, the association of these same risk factors with cognitive decline may increase awareness of the need to acquire healthy habits from the earliest stages of life.”
Moreover, the results provide yet more support for the importance of implementing primary cardiovascular prevention strategies in middle age as a valuable therapeutic approach to slowing or even halting brain alterations that could contribute to future cognitive decline.
The advanced stages of vascular disease and dementia often occur together, but until now this association has not been documented at earlier stages. The CNIC-coordinated study, led by Dr. Marta Cortés Canteli, shows that in middle age, years before any clinical signs appear, atherosclerosis and cardiovascular risk factors already show an association with low metabolism in the brain regions that are implicated in the future development of dementia, especially Alzheimer disease.
Using advanced imaging by positron emission tomography (PET), the research team quantified brain metabolism in more than 500 participants in the PESA-CNIC-Santander study. The participants had an average age of 50 years and no symptoms, but already had evidence of atherosclerosis in their arteries.
“We found that a higher cardiovascular risk in apparently healthy middle-aged individuals was associated with lower brain metabolism in parietotemporal regions involved in spatial and semantic memory and various types of learning,” said Dr. Cortés Canteli. Dr. Juan Domingo Gispert, head of the Neuroimaging group at the BBRC, noted that “the brain areas showing low metabolism in participants with higher cardiovascular risk are the same areas affected in Alzheimer disease, suggesting that these individuals may have higher than normal vulnerability to this disease.”
The study is the largest of its type to date in a healthy middle-aged population and could signal a paradigm change in the understanding of the links between vascular and brain disease, say the authors.
Among the modifiable cardiovascular risk factors most closely associated with a reduction in brain metabolism, the investigators saw the biggest effect with hypertension. “We found that the same risk factors that damage the heart and the large arteries, and especially hypertension, are closely linked to the decline in brain metabolism years before the appearance of symptoms,” said Dr. Fuster.
The research team also found that a higher number of plaques in the carotid arteries, which carry blood to the brain, was associated with lower brain metabolism in areas of the limbic system and the parietal lobe, both of which are intimately linked to the development of Alzheimer disease.
“The next step will be to determine whether individuals with subclinical atherosclerosis in the carotid arteries and low brain metabolism at the age of 50 go on to experience cognitive decline 10 years later,” said Dr. Cortés Canteli.
The PESA study is cofinanced equally by the CNIC and Santander Bank. PESA receives additional funding from the Instituto de Salud Carlos III, Madrid, Spain (ISCIII, PI15/02019, PI17/00590 & PI20/00819), the European Regional Development Fund (ERDF - A Way to Build Europe), and the European Social Fund (ESF - Investing in Your Future ). The CNIC is supported by the ISCIII, the MCIN and the Pro-CNIC Foundation. The BBRC is financed mainly by the Fundación “la Caixa”, the EU/EFPIA (European Federation of Pharmaceutical Industries and Associations) Innovative Medicines Initiative Joint Undertaking EPAD, and the Innovative Medicines Initiative 2 Joint Undertaking. This joint project was supported by the European Union Horizon 2020 research and innovation programme and the EFPIA.
Cortes-Canteli, M., Domingo Gispert, J., Salvado, G., Toribio-Fernandez, R., Tristao-Pereira, C., Falcon, C., Oliva, B., Mendiguren, J., Fernandez-Friera, L., Sanz, J., Garcia-Ruiz, J. M., Fernandez-Ortiz, A., Sanchez-Gonzalez, J., Ibanez, B., Luis Molinuevo, J., & Fuster, V. (2021). Subclinical Atherosclerosis and Brain Metabolism in Middle-Aged Individuals The PESA Study. Journal of the American College of Cardiology, 77(7), 888-898. https://doi.org/10.1016/j.jacc.2020.12.027
Journal of the American College of Cardiology
CNIC researchers explain how high blood pressure, the most important cause of disease worldwide, accelerates atherosclerosis
CNIC researchers explain how high blood pressure, the most important cause of disease worldwide, accelerates atherosclerosis
High blood pressure, the most important cause of disease worldwide, accelerates atherosclerosis, but the mechanism is unknown. Using genetically modified minipigs, researchers from the CNIC and Aarhus University (Denmark), have demonstrated that high blood pressure alters the structure of arteries, leading to more accumulation of LDL cholesterol and faster development of atherosclerosis.
Blood pressure-lowering drugs are routinely used to prevent the development of atherosclerosis and heart disease, but the mechanism of this effect is still unknown. People suffering from high blood pressure (hypertension) often have accompanying changes in the hormones that control blood pressure and it has been unclear whether the pressure itself or the hormonal changes are the driver of accelerated atherosclerosis.
To investigate this, the researchers analyzed the development of atherosclerosis in minipigs that were genetically engineered to have high blood cholesterol and develop atherosclerosis.
“Minipigs have arteries that are very similar in structure to human arteries and like humans they develop atherosclerosis in the heart when exposed to high blood cholesterol”, said the study coordinator Dr. Jacob Fog Bentzon. As is also the case in humans, the development of the early stages of the disease is asymptomatic, and therefore experiments on atherosclerosis can be conducted in minipigs with high animal welfare.
By manipulating blood pressure in the pigs and by analyzing the effects on arteries in the heart, the researchers found that the direct forces of pressure on arteries leads to structural changes that facilitate the development of atherosclerosis. “Arteries become denser and allow less passage of molecules from the blood. This includes the LDL particles that carry blood cholesterol, which instead accumulate in the innermost layer of arteries, where they drive the development of atherosclerosis”, Dr. Jacob Fog Bentzon explained.
This finding uncovers an intimate relationship between the most important risk factors for atherosclerosis, LDL cholesterol and high blood pressure. While it has been known for decades that accumulation of LDL particles in arteries leads to atherosclerosis, the new research shows that high blood pressure accelerates the accumulation of LDL. Therefore, high blood pressure aggravates the effect of having high LDL cholesterol in the blood.
The new insight supports the need to keep both LDL cholesterol and blood pressure low throughout life by healthy diet choices, weight control, exercise, and, when needed, drug therapy. “It could also pave the way to the development of more effective therapies to offset the detrimental effects of hypertension on atherosclerosis”, concluded Dr. Jacob Fog Bentzon.
This project was a collaboration among the Experimental Pathology of Atherosclerosis and the Cardiovascular Proteomics groups at CNIC, CIBER Cardiovascular Diseases, and the Atherosclerosis Research Unit at Aarhus University in Denmark.
This work at CNIC was supported by grants from the Ministerio de Economia, Industria y Competividad with cofunding from the Fondo Europeo de Desarrollo Regional (SAF2016-75580-R and PGC2018- 097019-B-I00), the Instituto de Salud Carlos III-Fondo de Investigación Sanitaria (IPT17/0019–ISCIII-SGEFI/ERDF, ProteoRed), the Fundació la Marató de TV3 (grant 122/C/2015) and “la Caixa” Foundation (project code HR17-00247).
Al-Mashhadi RH, Al-Mashhadi AL, Nasr ZP, Mortensen MB, Lewis EA, Camafeita E, Ravlo K, Al-Mashhadi Z, Kjær DW, Palmfeldt J, Bie P, Jensen JM, Nørgaard BL, Falk E, Vázquez J, Bentzon JF. (2021). Local Pressure Drives Low-Density Lipoprotein Accumulation and Coronary Atherosclerosis in Hypertensive Minipigs. Journal of the American College of Cardiology, 77(5), 575-589. DOI: 10.1016/j.jacc.2020.11.059
Journal of the American College of Cardiology
CNIC scientists identify mutations acquired by blood cells that accelerate heart failure progression
CNIC scientists identify mutations acquired by blood cells that accelerate heart failure progression
A team of scientists at the CNIC and the Hospital Universitario Virgen de Arrixaca in Murcia has discovered that clonal hematopoiesis increases the risk of rapidly progressing heart failure, one of the chief causes of death in the world.
The adult human body produces hundreds of billions of blood cells every day. This essential process unavoidably leads to the appearance of mutations in the DNA of the progenitor cells. These are known as somatic mutations because they are acquired, not inherited. While most of these mutations are innocuous, occasionally a mutation gives affected cells a competitive advantage that allows them to expand progressively, generating clonal populations of blood cells. This phenomenon is known as clonal hematopoiesis.
Clonal hematopoiesis is linked to aging, because over time there is an increasing chance that a culprit mutation will be produced, explained Dr. José Javier Fuster, coordinator of the study published today in The Journal of the American College of Cardiology (JACC).
The study shows that clonal hematopoiesis is an important pathological process that accelerates and aggravates the clinical progression of heart failure, independently of the presence of atherosclerosis.
In this study, which included input from the CNIC Genomics and Bioinformatics Units and from investigators at Hospital Universitari Germans Trias i Pujol in Badalona (Barcelona), the research team analyzed how the presence of mutations linked to clonal hematopoiesis affects the clinical progression of patients with ischemic or non-ischemic heart failure.
For the researchers, these findings “demonstrate the importance of clonal hematopoiesis as a pathogenic process that accelerates and aggravates heart failure progression, independently of the presence of atherosclerosis.”
The authors conclude that their study supports the emerging idea that “clonal hematopoiesis represents a new cardiovascular risk factor and an important link between aging and cardiovascular disease.” The results, moreover, “open the way to the development of personalized therapies for patients with these somatic mutations, with the aim of preventing heart failure progression.”
This study was funded by a Beca Leonardo para Investigadores y Creadores Culturales (2019) from the Fundación BBVA, the Carlos III Institute of Health, the Spanish Ministry of Science and Innovation, and the Fundación Séneca de Ciencia y Tecnología de la Región de Murcia. José Javier Fuster is a member of the Transatlantic Network on “Clonal hematopoiesis and atherosclerosis”, funded by the Leducq Foundation.
Pascual-Figal, D. A., Bayes-Genis, A., Diez-Diez, M., Hernandez-Vicente, A., Vazquez-Andres, D., de la Barrera, J., Vazquez, E., Quintas, A., Zuriaga, M. A., Asensio-Lopez, M. C., Dopazo, A., Sanchez-Cabo, F., & Fuster, J. J. (2021). Clonal Hematopoiesis and Risk of Progression of Heart Failure With Reduced Left Ventricular Ejection Fraction. Journal of the American College of Cardiology, 77(14), 1747-1759. https://doi.org/10.1016/j.jacc.2021.02.028
Nature Communications
Inhibition of proteins activated by nitric oxide reverses aortic aneurysm in Marfan syndrome
Inhibition of proteins activated by nitric oxide reverses aortic aneurysm in Marfan syndrome
Scientists at the CNIC and the Centro de Biología Molecular Severo Ochoa (CBM-CSIC-UAM) have discovered that the nitric oxide (NO) pathway is overactivated in the aortas of mice and patients with Marfan Syndrome and that the activity of this pathway causes the aortic aneurysms that characterize this disease.
The results of the study, published in Nature Communications, reveal the essential role played by NO in Marfan Syndrome aortic disease and identify new therapeutic targets and markers of NO pathway activation that could be used to monitor disease status and progression.
Aortic aneurysm (AA) is a progressive dilatation and weakening of the aortic wall. AA can be harmless, but in some patients can lead to dissection (rupture) of the aorta, resulting in death.
The study identifies new biomarkers associated with this syndrome that have the potential to improve the clinical treatment and prognosis of Marfan Syndrome patient’s syndrome.
Current treatments for Marfan Syndrome are aimed at reducing blood pressure on the artery wall, but do not prevent its deterioration. The only effective intervention for the aortic disease in Marfan Syndrome is surgery.
The researchers therefore recognize “the urgent need to identify new targets for the development of pharmacological treatments for thoracic aortic aneurysm and dissection (TAAD) in Marfan Syndrome.”
This study reveals the essential role played by NO in Marfan Syndrome aortic disease. “We previously detected high expression of a protein with a high capacity for NO production in the aortas of Marfan Syndrome patients and an mouse model of the disease, and we therefore undertook an in-depth investigation of the role of NO in the associated aortic disease,” explained lead investigator Juan Miguel Redondo.
“We observed that treatment of healthy mice with supra-pharmacological doses of an NO donor induced TAAD similar to that seen in Marfan mice. The NO donor treatment also reproduced the degeneration of the aortic wall, an essential step in the development of TAAD,” added Dr. Redondo. “Through these experiments, we showed that elevated production of NO is necessary and sufficient for the development of TAAD in Marfan Syndrome.”
Given this important role of NO in the development of TAAD, the researchers decided to focus on the enzymes soluble guanylate cyclase (sGC) and type I cGMP-dependent protein kinase (PRKG1), two NO-regulated proteins. “Our analysis detected elevated activities of both sGC and PRKG1 in samples from mice and patients with Marfan Syndrome,” said Dr. Redondo.
“We were able to completely reverse the aortic disease in Marfan mice by treating them with inhibitors of these two proteins or by genetically silencing the expression of Prkg1, demonstrating that the NO-sGC-PRKG1 pathway mediates the development of TAAD in Marfan Syndrome,” added Dr. Campanero.
Given the need for new pharmacological treatments for Marfan Syndrome aortic disease, “the results of this study open the way to the use of sGC and PRKG1 inhibitors in preclinical and clinical trials for this syndrome and possibly other aortic diseases,” said Dr. Redondo.
The research team also explored possible “footprints” left by high blood concentrations of NO.
“This discovery has important implications for patients with this syndrome, because these molecules could be used as biomarkers for disease monitoring, and we are now studying their potential as prognostic indicators,” explained Dr. Redondo.
This study was funded by the Fundación “la Caixa” through the CaixaResearch Call for Health Projects, the Ministerio de Ciencia e Innovación and Ministero de Universidades, the Comunidad de Madrid, the CSIC, the Marfan Foundation, the Fundación La Marató and the CIBER Cardiovascular Diseases (CIBER-CV) Instituto de Salud Carlos III.
de la Fuente-Alonso, A., Toral, M., Alfayate, A., Jesus Ruiz-Rodriguez, M., Bonzon-Kulichenko, E., Teixido-Tura, G., Martinez-Martinez, S., Jose Mendez-Olivares, M., Lopez-Maderuelo, D., Gonzalez-Valdes, I., Garcia-Izquierdo, E., Mingo, S., Martin, C. E., Muino-Mosquera, L., De Backer, J., Francisco Nistal, J., Forteza, A., Evangelista, A., Vazquez, J., … Redondo, J. M.(2021). Aortic disease in Marfansyndrome is caused by overactivation of sGC-PRKG signaling by NO. Nature Communications, 12(1), 2628. https://doi.org/10.1038/s41467-021-22933-3
JOURNAL FOR IMMUNOTHERAPY OF CANCER
CNIC scientists discover a new strategy to improve cancer immunotherapy
CNIC scientists discover a new strategy to improve cancer immunotherapy
Scientists at the CNIC have designed a new strategy to potentiate immunotherapy. In the study, published in the Journal for Immunotherapy of Cancer, the team led by Dr. David Sancho identifies a mechanism through which dead tumor cells stall the response of the immune system, reducing the antitumor capacity of immune cells that attack cancers.
The scientists improved the efficacy of cancer immunotherapy through an approach that combines blocking antibodies with cytokines or chemotherapy treatments that promote the development of dendritic cells. The team believe that this approach can be transferred to the clinic to optimize cancer immunotherapy.
Unfortunately, not all patients benefit from immunotherapy, because many cancers develop mechanisms to evade the immune system. One of these mechanisms consists of impeding the migration of immune cells to the tumor microenvironment.
The type of immune cells that enter the tumor has a major influence on cancer patient survival. Survival is higher after infiltration by CD8 T cells, which eliminate tumor cells, and by dendritic cell subtypes that attract and activate CD8 T cells. Scientists in the field have therefore focused their efforts on developing methods to increase the numbers of these cells in the tumor microenvironment.
Immune cells have an extensive repertoire of receptors through which they interact with their environment, allowing them to recognize pathogens and also detect tissue damage. Study coordinator Dr. David Sancho’s team recently showed that the detection of dead cells by the receptor DNGR-1, expressed on dendritic cells, prevents excessive inflammation. In cancer, this action can cause more harm than good. “High immune-cell infiltration of the tumor microenvironment will promote tumor elimination by the immune system,” said Dr. Sancho.
In the study, the scientists found that the recognition of dead tumor cells through DNGR-1 expressed on dendritic cells prevents further infiltration of both dendritic cells and the tumor-eliminating CD8 T cells, thus preventing the immune system from attacking the tumor. The researchers managed to increase the efficiency of antitumor immunotherapy by using blocking antibodies in combination with cytokines or chemotherapy to promote dendritic cell development.
The research shows that the antitumor activity of dendritic cells can be promoted through targeted interventions. The first of these is the administration of the cytokine Flt3L, and the second is antibody blockade of DNGR-1. Flt3L acts as a ‘stimulant’, increasing the numbers of dendritic cells and promoting their entry into the tumor microenvironment. The team also found that circulating levels of Flt3L can be increased by stimulating the body’s own production of the cytokine with specific chemotherapy treatments.
This new study shines a light on some of the mechanisms that promote correct tumor infiltration by antitumor immune cells and proposes a new strategy to potentiate this infiltration and antitumor immunotherapy. The study thus identifies a new tool for combating cancer by strengthening the body’s own defense.
The study was funded by the Fundación “la Caixa”, the Asociación Española contra el Cáncer, the National Institute for Health Research Manchester Biomedical Research Center, the European Research Council (ERC-2016-Consolidator Grant 725091), the European Commission (635122-PROCROP H2020), the Ministerio de Ciencia, the Agencia Estatal de Investigación, the European Regional Development Fund (ERDF) (SAF2016-79040-R), the IMMUNOTHERCAN de la Comunidad de Madrid, a FIS-Instituto de Salud Carlos III grant, the Fundación Acteria, Atresmedia (Constantes y Vitales), and Fundació La Marató de TV3.
Cueto, F. J., Del Fresno, C., Brandi, P., Combes, A. J., Hernandez-Garcia, E., Sanchez-Paulete, A. R., Enamorado, M., Bromley, C. P., Gomez, M. J., Conde-Garrosa, R., Manes, S., Zelenay, S., Melero, I., Iborra, S., Krummel, M. F., & Sancho, D. (2021). DNGR-1 limits Flt3L-mediated antitumor immunity by restraining tumor-infiltrating type I conventional dendritic cells. Journal for Immunotherapy of Cancer, 9(5), e002054. https://doi.org/10.1136/jitc-2020-002054
JNEW ENGLAND JOURNAL OF MEDICINE
The first blood biomarker to distinguish between myocarditis and acute myocardial infarction
The first blood biomarker to distinguish between myocarditis and acute myocardial infarction
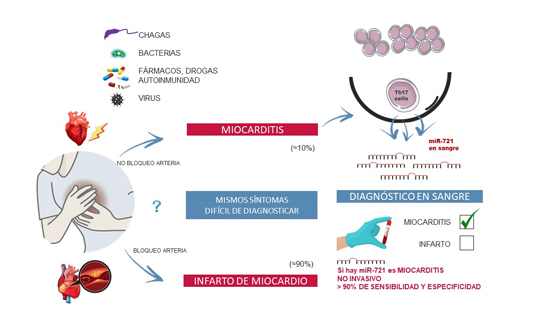
Scientists at the CNIC have identified the first blood biomarker for myocarditis, a cardiac disease that is often misdiagnosed as myocardial infarction.
The study, led by Dr. Pilar Martín and published in The New England Journal of Medicine, has detected the presence of the human homolog of micro RNA miR-721 in the blood of myocarditis patients.
These results are of paramount importance because they establish the first validated blood marker for myocarditis with high sensitivity and specifity (>90%). This will allow clinicians to distinguish between this disease and other cardiomyopathies like acute myocardial infarction, myocardial infarction with nonobstructive coronary arteries (MINOCA), and other inflammatory diseases with an autoimmune origin.
“Our finding has great potential as a valuable clinical tool for the precise and noninvasive diagnosis of myocarditis from small drops of blood,” said Dr. Martín.
The diagnosis of myocarditis is challenging, and the availability of a sensitive and specific marker of acute myocardial inflammation could have a major clinical impact, improving the diagnosis of myocarditis both generally and particularly in its early phases.
Myocarditis is an inflammatory disease of the heart caused by infection, toxins, drugs, or autoimmune disorders. If untreated, myocarditis can progress to potentially fatal dilated cardiomyopathy, requiring heart transplantation.
The prevalence of myocarditis remains uncertain because it is often difficult to achieve a confirmed diagnosis.
Myocarditis is usually diagnosed after coronary angiography or computed tomography scans have discarded coronary artery disease, followed by confirmation of the diagnosis by magnetic resonance imaging (MRI).
However, not all centers have access to MRI technology, and the current gold standard for myocarditis diagnosis is endomyocardial biopsy, an invasive procedure normally reserved for severe cases. There is thus a pressing clinical need for the development of reliable and accessible tools for the early diagnosis of acute myocarditis.
Moreover, myocarditis is a side effect of cancer therapy with immunotherapy drugs called “immune checkpoint inhibitors”.: Although rare, this effect can have serious consequences for patients.
There are currently no specific markers for the diagnosis of patients susceptible to developing myocarditis during cancer immunotherapy.
The researchers are currently designing studies to evaluate the potential of the biomarker as a predictor of short-term and long-term risk; the persistence of myocardial inflammation, and the risk of relapse, clinical progression, and adverse ventricular remodeling.
The CNIC is the sole owner of a patent related to the biomarker and its use for the diagnosis of myocarditis. The CNIC is now exploring licensing agreements with industrial partners to develop and commercialize this technology in order to make it available for clinical use.
This study received funding from the Ministerio de Ciencia e Innovación(MICINN) through the Instituto de Salud Carlos III (ISCIII)-Fondo de Investigación Sanitaria; the CIBER-CV; the Comunidad de Madrid; a Fundación BBVA Beca Leonardo award; Fundació La Marató TV3; and European Research Council grants ERC-2011-AdG 294340-GENTRIS to F.S-M. and ERC-2018-CoG 819775-MATRIX to B.I.
Blanco-Dominguez, R., Sanchez-Diaz, R., de la Fuente, H., Jimenez-Borreguero, L. J., Matesanz-Marin, A., Relano, M., Jimenez-Alejandre, R., Linillos-Pradillo, B., Tsilingiri, K., Martin-Mariscal, M. L., Alonso-Herranz, L., Moreno, G., Martin-Asenjo, R., Garcia-Guimaraes, M. M., Bruno, K. A., Dauden, E., Gonzalez-Alvaro, I., Villar-Guimerans, L. M., Martinez-Leon, A., … Martin, P. (2021). A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. New England Journal of Medicine, 384(21), 2014-2027.
Nature Communications
CNIC scientists identify essential factors for limb formation
CNIC scientists identify essential factors for limb formation
Scientists at the CNIC, working in partnership with researchers at the Institut de Recherches Cliniques de Montréal (IRCM) in Canada, have identified Meis transcription factors as essential biomolecules for the formation and antero-posterior patterning of the limbs during embryonic development.
In the study, published in Nature Communications, the research team carried out an in-depth characterization of the Meis family of transcription factors. Genetic deletion of all four family members showed that these proteins are essential for the formation of the limbs during embryonic development. “An embryo that develops in the absence of Meis does not grow limbs,” said study coordinator Miguel Torres, who leads the Genetic Control of Organ Development and Regeneration group at the CNIC.
Embryonic development is a highly complex process involving interactions among a large array of molecules to ensure the correct formation of a specific organ or tissue from a small initial number of cells. The limbs, explained first author Irene Delgado, “start to form as bulges called limb buds on the flank of the embryo. Growth of the limb bud eventually results in the formation of the skeletal components of the limb.”
One of the factors that plays a crucial role in the developing limb is a group of transcription factors called Meis. “In a normal embryo, the Meis genes are expressed very early during the formation of the limb buds,” Delgado explained.
In this study, in-depth molecular characterization of developing mouse embryos revealed that Meis factors initiate a signaling cascade that is essential for limb bud development and involves contributions from Fgf10 and Lef1. “Our results identify roles for Meis transcription factors in the developing limb and reveal their participation in essential pathways for limb development. During early limb bud formation, Meis transcription factors are essential for inducing the expression of Fgf10 and Lef1, continued Delgado.”
An embryo that lacks Meis genes is unable to grow limbs, but the presence of just one of these four genes (a single allele) “is enough to initiate limb development and also reveals other functions of Meis, such as its importance for the formation of the proximal limb structures (pelvis and femur) and for antero-posterior limb patterning,” noted Torres.
Nevertheless, the pelvis and femur of embryos with a single Meis allele are smaller than those of a normal embryo. Moreover, added Delgado, “These embryos have defects in, or simply lack, posterior skeletal elements such as the fibula and posterior digits.”
The authors further demonstrated that the molecular basis for these defects is failed initiation of the expression of the Sonic Hedgehog gene, which is essential for antero-posterior limb patterning.
The study was supported by grant PGC2018-096486-B-I00 from the Spanish Ministerio de Ciencia e Innovación.
Delgado, I., Giovinazzo, G., Temino, S., Gauthier, Y., Balsalobre, A., Drouin, J., & Torres, M. (2021). Control of mouse limb initiation and antero-posterior patterning by Meis transcription factors. Nature Communications, 12(1), 3086. https://doi.org/10.1038/s41467-021-23373-9
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
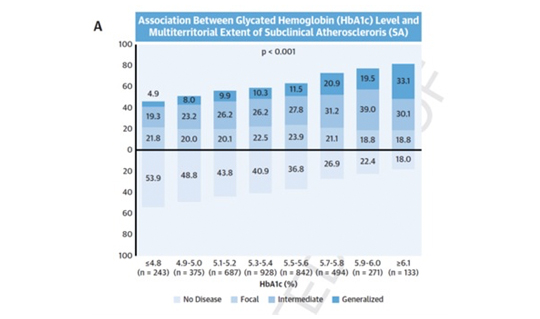
A blood sugar biomarker identifies patients with atherosclerosis and a risk of cardiovascular events
A blood sugar biomarker identifies patients with atherosclerosis and a risk of cardiovascular events
The routine use of the glycosylated hemoglobin test to track blood sugar levels in the general population can identify individuals with more advanced atherosclerotic disease. Currently used in the diagnosis and management of diabetes, glycosylated hemoglobin can provide a useful estimate of atherosclerotic disease, and therefore of cardiovascular risk, in individuals without diabetes may or may not have prediabetes.
The advance heralded by the CNIC study is the use of this blood-sugar measure in apparently healthy middle-aged individuals who do not have diabetes mellitus but with or without possible prediabetes.
When used in combination with traditional risk factors (hypertension, dyslipidemia, and smoking), the glycosylated hemoglobin test more accurately distinguishes between people at high and low risk of atherosclerotic disease.
The study, published in The Journal of American College of Cardiology (JACC), proposes that, because glycosylated hemoglobin levels can easily be reduced through lifestyle changes, the test should be at the front line of risk reduction strategies.
The glycosylated hemoglobin diagnostic test is cheap, accessible, and widely used in daily clinical practice, explained Dr. Xavier Rosselló, CNIC scientist and cardiologist at Hospital Universitario Son Espases in Palma de Mallorca. The test can therefore be put to immediate use to calculate the degree of subclinical atherosclerosis in the general population.
The study forms part of the collaborative PESA-CNIC-SANTANDER project.
The new CNIC finding provides a simple way to increase the accuracy of cardiovascular risk ranking in individuals without diabetes and with or without prediabetes.
The authors conclude that “this study establishes glycosylated hemoglobin as a mass-use biomarker because its greatest benefit is in individuals at low cardiovascular risk, who form the immense majority of the general population and account for most cardiovascular deaths in absolute terms.”
The study was funded by the Instituto de Salud Carlos III (ISCIII, PI15/ 02019, PI17/00590, PI20/00819) and the European Regional Development Fund (ERDF) “A way to make Europe” and included scientists from the CIBER-CV research network.
Rossello, X., Raposeiras-Roubin, S., Oliva, B., Sanchez-Cabo, F., Garcia-Ruiz, J. M., Caimari, F., Mendiguren, J. M., Lara-Pezzi, E., Bueno, H., Fernandez-Friera, L., Fernandez-Ortiz, A., Sanz, J., Ibanez, B., & Fuster, V. (2021). Glycated Hemoglobin and Subclinical Atherosclerosis in People Without Diabetes. Journal of the American College of Cardiology, 77(22), 2777-2791. https://doi.org/10.1016/j.jacc.2021.03.335
ACS Nano
CNIC scientists describe a possible disease-causing mechanism in hypertrophic cardiomyopathy
CNIC scientists describe a possible disease-causing mechanism in hypertrophic cardiomyopathy
Scientists at the CNIC have described a potential disease-causing mechanism in hypertrophic cardiomyopathy (HCM), the most frequent hereditary disease of the heart. The study, published in the journal ACS Nano, provides the first description of an association between this disease and mechanical alterations to a component of the heart’s contractile machinery.
The heart muscle is under constant mechanical stress throughout life as it contracts to pump blood to the body. The laboratory led by Dr. Jorge Alegre-Cebollada investigates how the mechanical properties of the cardiac proteins determine the physiological behavior of this muscle and how alterations to these properties lead to the appearance of diseases like HCM. In this disease, the left ventricle becomes enlarged, and severe manifestations include heart failure and sudden death.
Scientists have known for more than 20 years that HCM is caused by mutations in proteins with a mechanical function in the heart. One of the challenges of cardiovascular genetics is to identify which among the genetic variants found in patients and their families cause disease. Knowing if a mutation is disease-causing or not is important because this information will determine the clinical follow-up of family members and, potentially, their treatment.
The study, coordinated by Dr. Alegre-Cebollada, analyzed cardiac myosin-binding protein C (cMyBP-C), the most frequently mutated protein in HCM patients. “A high proportion of mutations in the cMyBP-C gene cause amino-acid changes in the protein; however, the mechanisms by which these mutations cause HCM are not precisely known.”
Dr. Alegre-Cebollada’s group, in close partnership with clinical and molecular researchers in Europe and the US, set up a database of cMyBP-C variants with a clear link to HCM in order to define the molecular defects underlying the disease.
Using bioinformatics and experimental approaches, the research team discovered that around half of these mutations affect the integrity of cMyBP-C messenger RNA (mRNA) or protein. These results have already been accepted for publication in the Journal of Biological Chemistry and have been the subject of a commentary article in the leading medical genetics journal Genetics in Medicine.
While alterations to mRNA or protein integrity could explain the pathogenicity of half the mutations analyzed in the earlier study, Dr. Alegre pointed out that the other half do not cause disease via this route.
Using advanced biophysical techniques based on atomic force microscopy, in the new study the team showed that some of the disease-causing mutations in cMyBP-C produce defects in the mechanical properties of the protein that can alter the contractile function of cardiomyocytes in HCM patients.
Identifying the molecular mechanisms underlying HCM is essential for determining which cMyBP-C mutations cause the disease. This knowledge is therefore also crucial for the clinical follow-up and possible treatment of patients and their families, said the authors.
The study was funded by the Ministerio de Ciencia e Innovación, the European Research Area Network on Cardiovascular Diseases (MINOTAUR consortium, through the Instituto de Salud Carlos III), the Comunidad de Madrid, the US National Institutes of Health, the government of the Basque region, the Italian Ministry of Education, Universities and Research, and postdoctoral fellowships from the School of Medicine and the Maternal and Child Health Institute at Stanford University. The research team also included scientists from the Cardiovascular Biomedical Research Network (CIBER-CV).
Suay-Corredera, C., Pricolo, M. R., Velazquez-Carreras, D., Pathak, D., Nandwani, N., Pimenta-Lopes, C., Sanchez-Ortiz, D., Urrutia-Irazabal, I., Vilches, S., Dominguez, F., Frisso, G., Monserrat, L., Garcia-Pavia, P., de Sancho, D., Spudich, J. A., Ruppel, K. M., Herrero-Galan, E., & Alegre-Cebollada, J. (2021). Nanomechanical Phenotypes in Cardiac Myosin-Binding Protein C Mutants That Cause Hypertrophic Cardiomyopathy. Acs Nano, 15(6), 10203-10216. https://doi.org/10.1021/acsnano.1c02242
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
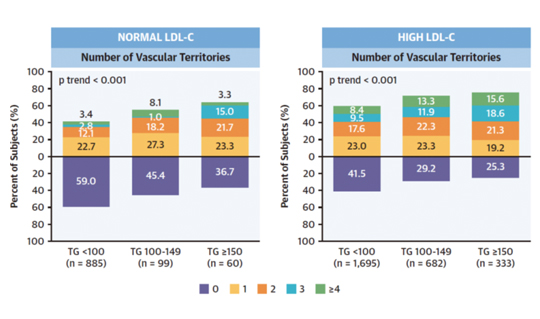
Spanish scientists provide the first demonstration that triglycerides are a primary risk factor for atherosclerosis
Spanish scientists provide the first demonstration that triglycerides are a primary risk factor for atherosclerosis
Triglycerides can be as important an indicator of cardiovascular risk as high cholesterol. A study conducted by researchers at the CNIC shows for the first time that hypertriglyceridemia is associated with subclinical atherosclerosis and vascular inflammation in individuals with low-to-moderate cardiovascular risk, even if they have normal circulating concentrations of LDL-C, known as ‘bad’ cholesterol. The results are published in The Journal of American College of Cardiology (JACC).
Until now, triglycerides have been considered a secondary factor in the origin of atherosclerosis, far less important than cholesterol, especially cholesterol bound to low-density lipoproteins (LDL). If, “if LDL-C concentrations are normal, current cardiovascular prevention guidelines do not recommend treatment for high circulating triglyceride concentrations unless the patient has a high cardiovascular risk,” said study first author Dr. Sergio Raposeiras-Roubin.
The new study provides the first demonstration that “in individuals with a low-to-moderate cardiovascular risk according to standard scores (the majority of the population), high circulating levels of triglycerides are associated with a greater risk of developing atherosclerosis, even among people with normal LDL-C.”
The new study forms part of the PESA CNIC-SANTANDER study (Progression and Early detection of Subclinical Atherosclerosis). The study, led by CNIC General Director Dr. Valentín Fuster, has demonstrated the high prevalence of subclinical atherosclerosis in the general population, establishing the importance of detecting the disease early in its silent phase.
Moreover, this article shows that triglyceride levels are associated not only with the presence of atherosclerosis, but also with vascular inflammation.
CNIC Clinical Research Director Dr. Borja Ibáñez explained that this result indicates a strong association between elevated circulating triglyceride levels and the early stages of atherosclerosis, a finding “with important implications for the design of prevention strategies.”
The results indicate that clinical practice guidelines should be modified to emphasize the need to control not only LDL-cholesterol but also triglyceride levels. “The measurement of blood triglycerides is routine, and fortunately there are abundant effective medicines available to ensure appropriate levels,” concluded Dr. Fuster.
The study received funding from the Carlos III Institute of Health and the European Regional Development Fund. Dr. Ibáñez’s research is supported by the European Research Council through the MATRIX project (ERC-COG-2018-ID: 819775).
Raposeiras-Roubin, S., Rossello, X., Oliva, B., Fernandez-Friera, L., Mendiguren, M. J., Andres, V., Bueno, H., Sanz, J., de Vega, V. M., Abu-Assi, E., Iniguez, A., Fernandez-Ortiz, A., Ibanez, B., & Fuster, V. (2021). Triglycerides and Residual Atherosclerotic Risk. Journal of the American College of Cardiology, 77(24), 3031-3041. https://doi.org/10.1016/j.jacc.2021.04.059
JACC-CARDIOVASCULAR IMAGING
CNIC and Philips develop ultrafast cardiac magnetic resonance technology that analyzes the heart in less than 1 minute
CNIC and Philips develop ultrafast cardiac magnetic resonance technology that analyzes the heart in less than 1 minute
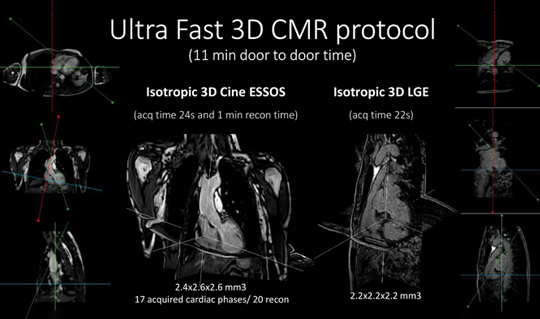
Scientists at the CNIC and Philips have developed a revolutionary technology that can perform cardiac magnetic resonance (CMR) scans in under a minute. ESSOS (Enhanced SENSE by Static Outer volume Subtraction) allows precise assessment of heart anatomy and function, as well as reducing healthcare costs and increasing patient comfort. The new methodology has been tested on more than 100 patients with a range of heart conditions. The results have been published in JACC: Cardiovascular Imaging, the world-leading journal in the field of cardiac imaging.
CMR provides a noninvasive and radiation-free method for exploring the heart and is the ideal technique for studying heart anatomy, function, and even cell composition. Although most hospitals have magnetic resonance scanners, these are not often used for heart studies because a complete CMR study takes so long. Study first author Dr. Sandra Gómez-Talavera, a CNIC investigator and cardiologist at Fundación Jiménez Díaz University Hospital, explained that “a complete CMR study takes 45-60 minutes, and many patients don’t go through with it because remaining in the scanner for this long is too uncomfortable.”
In addition, hospital magnetic resonance scanners are needed for other studies, limiting their availability for long-duration cardiac assessments.
To overcome these obstacles to CMR, CNIC scientists working in partnership with Philips have developed a technique for accelerated CMR acquisition. The technique, explained Dr. Gómez-Talavera, “allows the study of the anatomy and function (The motility) of the heart muscle, as well as infarcted and fibrotic tissue. The method can be used to study the whole thoracic cavity in 3D, with algorithms used to focus exclusively on the heart and major vessels (the mobile elements), reducing the scan time.”
“We have demonstrated in a large group of patients that CMR with this technology yields the same information as the standard technique, but at less than 10% of the patient scan time,” said CNIC Clinical Research Director Dr. Borja Ibáñez, a cardiologist at Fundación Jiménez Díaz University Hospital and a CIBER-CV group leader. Dr. Ibáñez is one of the two lead authors on the study.
ESSOS is protected by a patent held jointly by the CNIC and Philips and is the fruit of almost 10 years’ collaboration. The research team believe that this new technology will revolutionize cardiac imaging.
ESSOS allows images to be obtained 34 times faster than with the standard technology in current use. “All the information needed to understand heart form and function can be acquired in a little over 20 seconds,” noted Sánchez-González, adding that “a further 20-second acquisition is all that is needed to detect infarction or fibrosis. This brings the scan to an end, in less than 1 minute.”
A key advantage of the technology is that it can be used with the magnetic resonance scanners already installed in hospital.
This research was funded by the Instituto de Salud Carlos III through a FIS technology development grant, a Spanish Society of Cardiology Translational Research award, the European Research Council (ERC), and the Comunidad de Madrid (Red Madrileña de Nanomedicina en Imagen Molecular).
Gomez-Talavera, S., Fernandez-Jimenez, R., Fuster, V., Nothnagel, N. D., Kouwenhoven, M., Clemence, M., Garcia-Lunar, I., Gomez-Rubin, M. C., Navarro, F., Perez-Asenjo, B., Fernandez-Friera, L., Calero, M. J., Orejas, M., Cabrera, J. A., Desco, M., Pizarro, G., Ibanez, B., & Sanchez-Gonzalez, J. (2021). Clinical Validation of a 3-Dimensional Ultrafast Cardiac Magnetic Resonance Protocol Including Single Breath-Hold 3-Dimensional Sequences. Jacc-Cardiovascular Imaging, 14(9), 1742-1754. https://doi.org/10.1016/j.jcmg.2021.02.031
Circulation Research
CCNIC scientists describe one of the mechanisms underlying cardiac hypertrophy
CCNIC scientists describe one of the mechanisms underlying cardiac hypertrophy
CNIC Scientists have identified a new mechanism involved in the regulation of cardiac hypertrophy. The mechanisms underlying this disease remain largely unknown, and this situation is impeding the development of effective treatments.
The findings, published in Circulation Research, may spur the development of new tools for the treatment of cardiac hypertrophy, especially in Cushing syndrome patients.
The results, explained principal investigator Dr. Enrique Lara Pezzi, who leads the Molecular Regulation of Heart Failure group at the CNIC, reveal that the “The protein SRSF4 binds to and stabilizes the non-coding RNA GAS5, enabling it to block the action of the glucocorticoid receptor and thus prevent cardiac hypertrophy”.
The contractile function of the heart is enacted by cardiomyocytes. Although in the adult heart these cells have almost no ability to divide, Dr. Lara Pezzi explained that they have a remarkable ability to adapt to the changing demands placed on the heart.
One example of this capacity is the response to aortic stenosis. “With stenosis, the aortic valve cannot open fully, and the narrowing of the opening obliges the left ventricle to pump blood with greater force. This requires the heart muscle to contract more strongly,” continued Dr. Lara Pezzi.
Although cardiomyocytes cannot divide, Dr. Lara Pezzi explained that “to increase contractile capacity, these cells become enlarged, a process known as hypertrophy.”
However, while this response is effective at first, the thickening of the left ventricular wall (cardiac hypertrophy) triggers structural changes in the heart that cause a progressive loss of contractile capacity.
Continuing lack of knowledge about the mechanisms underlying cardiac hypertrophy is impeding the development of effective treatments. Dr. Lara-Pezzi’s group, in partnership with researchers at Hospital Puerta de Hierro in Majadahonda, the Spanish Cardiovascular Research Network (CIBER-CV), and the University of Frankfurt, has analyzed possible mechanisms underlying the development of this disease.
The researchers found that mice lacking the RNA-binding protein SRSF4 develop cardiac hypertrophy and have an impaired ability to relax the heart muscle, a condition known as diastolic dysfunction.
Further analysis showed that the absence of SRSF4 severely reduces the expression of the non-coding RNA GAS5. “SRSF4 binds directly to GAS5, preventing GAS5 degradation in the cell. GAS5 is an inhibitor of the glucocorticoid receptor, whose activation contributes to the development of cardiac hypertrophy,” explained Dr. Lara Pezzi.
First author, Dr. Javier Larrasa clarified that “the absence of SRSF4 triggers the degradation of GAS5, and this results in activation of the glucocorticoid receptor. In contrast, overexpression of GAS5 with a viral vector inhibits the glucocorticoid receptor and reduces cardiac hypertrophy.”
The research team concludes that the identification of the SRSF4–GAS5–glucocorticoid-receptor cell signaling pathway is a major advance in the characterization of the molecular mechanisms underlying cardiac hypertrophy and could serve as the basis for new treatments.
Dr. Lara Pezzi also commented that, the findings could be “particularly applicable to patients with Cushing syndrome, who develop cardiac hypertrophy in part through glucocorticoid receptor activation.”
An analysis of samples from Cushing syndrome patients revealed a degree of dysregulation of SRSF4 and GAS5 expression; however, confirming a role of this pathway in this disease will require further research.
This study received funding from the European Union (CardioNeT-ITN-289600 and CardioNext-ITN-608027), the Ministerio de Economía y Competitividad, and the Community of Madrid (2010-BMD-2321 “Fibroteam”). Funding was also provided by the Plan Estatal de I+D+I 2013-2016 and the European Regional Development Fund “A way to build Europe” program.
Larrasa-Alonso, J., Villalba-Orero, M., Marti-Gomez, C., Ortiz-Sanchez, P., Lopez-Olaneta, M. M., Ascension Rey-Martin, M., Sanchez-Cabo, F., McNicoll, F., Muller-McNicoll, M., Garcia-Pavia, P., & Lara-Pezzi, E. (2021). The SRSF4-GAS5-Glucocorticoid Receptor Axis Regulates Ventricular Hypertrophy. Circulation Research, 129(6), 669-683. https://doi.org/10.1161/CIRCRESAHA.120.318577
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
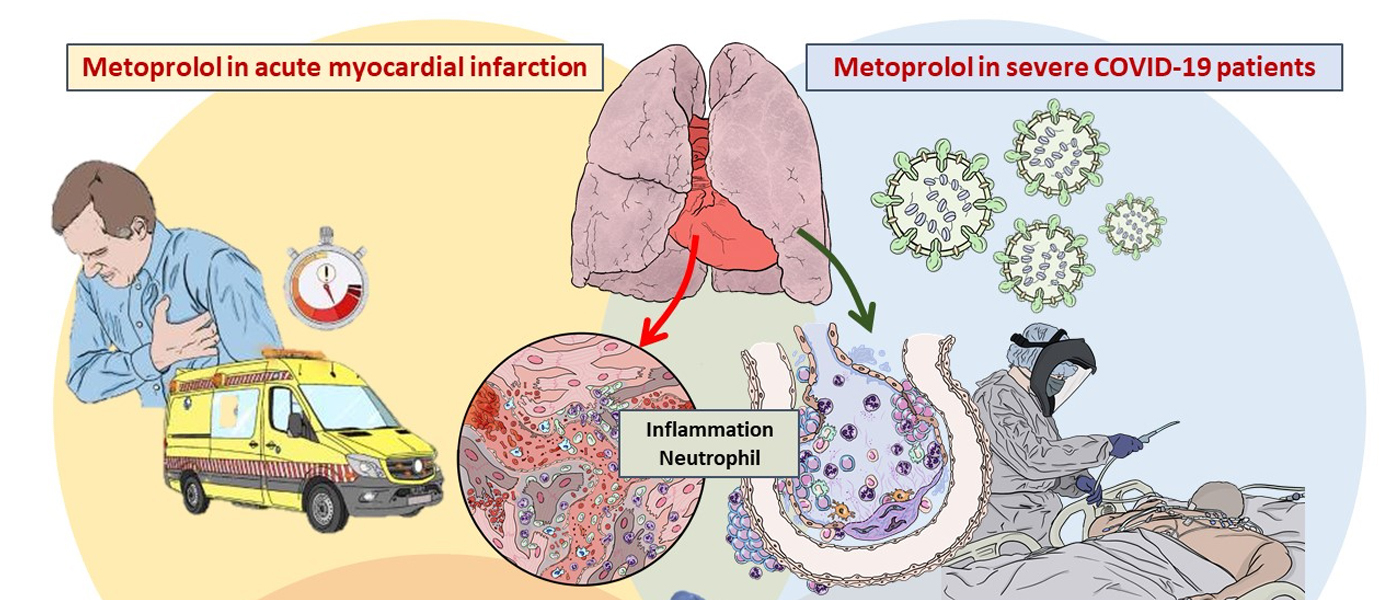
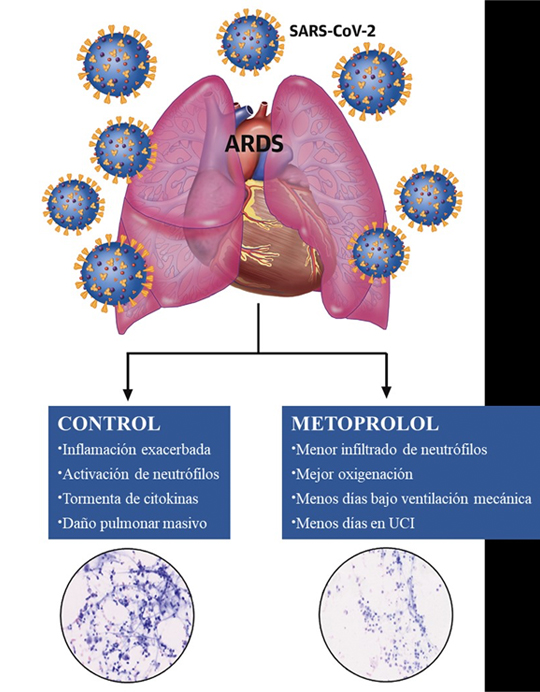
A drug costing less than €2 a day helps in the treatment of severely ill COVID-19 patients
A drug costing less than €2 a day helps in the treatment of severely ill COVID-19 patients
Metoprolol, a drug widely used to treat cardiovascular disease, is beneficial when administered to COVID-19 patients. This is the finding of a study by investigators at the CNIC, published in the Journal of American College of Cardiology (JACC).
The most severe form of COVID-19 is severe respiratory failure, which requires intubation and is associated with a high mortality rate. Pulmonary infection with the SARS-CoV2 virus can progress to acute respiratory distress syndrome (ARDS), in which inflammation and neutrophil hyperactivation play a central role. There is currently a lack of therapies for ARDS associated with COVID-19.
The study was led by Dr. Borja Ibáñez, group leader of the Translational Laboratory for Cardiovascular Imaging and Therapy at the CNIC, cardiologist at the Hospital Universitario Fundación Jiménez Díaz (FJD) in Madrid, and member of the CIBER-CV cardiovascular research network. The research team recently discovered that metoprolol, a well-established beta-blocker, has a highly selective effect on hyperactivated neutrophils during situations of acute stress such as a myocardial infarction. Given the central role played by neutrophils in ARDS, the team postulated that metoprolol might be an effective treatment for patients with severe COVID-19.
MADRID-COVID is a randomized clinical trial conducted in close collaboration between the CNIC and the cardiology, ICU, pulmonology, and biobank services at FJD Hospital. This pilot trial examined the effect of intravenous metoprolol administration on lung inflammation and respiratory function in severe COVID-19 patients intubated after developing ARDS.
The team “randomized 20 intubated COVID-19 patients to receive intravenous metoprolol (15 mg per day over 3 days) or to a control group that did not receive the drug. We analyzed the inflammatory infiltrate in bronchoalveolar fluid before and after treatment and also monitored clinical progression parameters such as oxygenation and days on mechanical ventilation.”
The intravenous metroprolol treatment significantly reduced neutrophil infiltration of the lungs and improved oxygenation.
Dr. Ibáñez added that “while we need to be cautious with these results of a pilot trial, we have observed that metoprolol treatment in this clinical setting is safe, is associated with a very significant reduction in lung infiltration, and appears to lead to very rapid improvements in patient oxygenation.”
The researchers therefore propose intravenous metoprolol as a “promising intervention that could improve the prognosis of severely ill COVID-19 patients.” They also emphasize that metoprolol is a safe and cheap drug (daily treatment cost below €2) that is readily available.
Joint first author Agustín Clemente-Moragón added that “the effect of metoprolol on the hyperactivation of inflammatory cells implicated in ARDS is exclusive to this beta-blocker.” In a previous experimental study, the same group recently demonstrated that other apparently similar beta-blockers have no effect on exacerbated lung inflammation.
The research team led by Dr. Ibáñez was awarded funding from the Instituto de Salud Carlos III (ISCIII) for a clinical trial to definitively demonstrate the clinical benefits of metoprolol in 350 ADRS patients admitted to 14 ICUs across Spain. The MAIDEN clinical trial will be coordinated by the cardiovascular CIBER research network and will include the participation of cardiovascular and respiratory specialists.
The study was partly funded by the European Comission (EERC-CoG grant Nº 819775) and the Ministerio de Ciencia e Innovación (MCN; ‘RETOS 2019’ grant Nº PID2019-107332RB-I00). The study was also supported by the Programa de Atracción de Talento de la Comunidad de Madrid.
Clemente-Moragón A, Martínez-Milla J, Oliver E, Santos A, Flandes J, Fernández I, Rodríguez-González L, Serrano Del Castillo C, Ioan AM, López-Álvarez M, Gómez-Talavera S, Galán-Arriola C, Fuster V, Pérez-Calvo C, Ibáñez B. (2021). Metoprolol in Critically Ill Patients With COVID-19. J Am Coll Cardiol, 78(10), 1001-1011. doi: 10.1016/j.jacc.2021.07.003
Scientific Reports
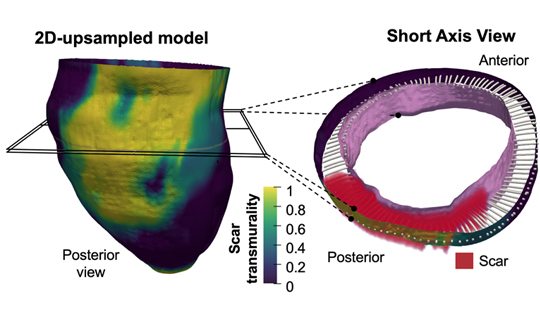
3D mapping of post-infarction scarring increases the prognostic potential of cardiac magnetic resonance imaging
3D mapping of post-infarction scarring increases the prognostic potential of cardiac magnetic resonance imaging
A multidisciplinary team of scientists based the CNIC and the Universidad de Valladolid, has developed a highly efficient method for identifying the 3-dimensional features of the scar tissue formed after a myocardial infarction. The study was carried out in partnership with scientists and clinicians at Hospital Clínico San Carlos, Hospital Universitario la Paz, Fundación Jiménez Díaz, Hospital Rúber Juan Bravo Quironsalud, Universidad Politécnica de Madrid, el Centro de Supercomputación de Barcelona, Philips healthcare Iberia, CIBER-CV1 and CIBER-BBN.
The new method allows 3-dimensional transmural (across the ventricular wall) mapping of scar tissue in the infarcted muscle. Transmural mapping of the infarcted tissue enables highly detailed characterization of the morphology of the damaged tissue and provides an accurate measure of infarct size relative to myocardial wall thickness, a parameter known as transmurality.
According to CNIC scientist David Filgueiras, “a major advantage of this new method is its full compatibility with standard cardiac magnetic resonance (CMR) sequences that take just a few minutes to acquire. This approach can thus significantly shorten the time needed for image acquisition, easing access to in-demand nuclear magnetic resonance scanners.”
“This novel methodology may provide an efficient approach in clinical practice after manual or automatic segmentation of myocardial borders in a small number of conventional 2D slices and automatic scar detection,” write the authors.
The results of the study show that low scar transmurality on CMR (below 10% of ventricular wall thickness for 3D sequences or 20% for 2D sequences) is associated with the clinical presentation of tachycardias involving infarcted ventricular tissue, known as ventricular tachycardias.
Describing the results study first authors Susana Merino, of Valladolid University, and Lilian Karina Gutiérrez, of the CNIC, said that the results reveal a significant correlation between low scar transmurality and the cardiac frequency of spontaneous ventricular tachycardia episodes.
The results also show that patients with low scar transmurality values had a higher probability of ventricular tachycardia recurrences during long-term follow-up.
The new method is especially promising because it uses conventional 2D delayed gadolinium-enhanced CMR sequences and requires only a limited number of slices. This technology is available at all centers that carry out CMR studies, and the method does not require 3D CMR studies.
The method was developed through technical, experimental, and clinical collaboration under the umbrella of a specific partnership between Valladolid University and the CNIC. Joint lead author Carlos Alberola explained that the technical advances of the method are built on procedures developed in the Image Processing Laboratory of the Escuela Técnica Superior de Ingenieros de Telecomunicación at Valladolid University.
“The first of these advances is a method for image interpolation that preserves topology. This allows high-resolution 3D images to be generated with a high level of precision from the images obtained with conventional CMR procedures.”
“A second advance is a mathematical method for characterizing fibrous tissue formed in the myocardial wall after an infarction. This tool provides a measure of the full 3D morphology of the scar relative to the thickness of the myocardium, a parameter known as transmurality.” The method uses a procedure based on partial differential equations to provide point-wise correspondences between the endocardium and the epicardium. These correspondences allow the definition of multiple indicators of the extent of the infarction.
The experimental arm of the study involved input from the Advanced Development in Arrhythmia Mechanisms and Therapy group at the CNIC, led by Dr. David Filgueiras. This group provided expertise in experimental models of myocardial infarction, essential for validating the methodology.
The CNIC laboratory also coordinated the study of the clinical application of the new method, in partnership with a multidisciplinary team of experts in the diagnosis and treatment of complex cardiac arrhythmias.
The study received funding from the Ministerio de Ciencia e Innovación (TEC2017-82408-R y PID2019-109329RB-I00); Fondo Europeo de Desarrollo Regional (CB16/11/00458); Heart Rhythm Association de la Sociedad Española de Cardiología; Fundación Interhospitalaria para la Investigación Cardiovascular (FIC); Fundación Eugenio Rodríguez Pascual; programa H2020 de la Unión Europea; el Ministerio de Asuntos Económicos y Transformación Digital, and the Fundación Carolina-BBVA.
Merino-Caviedes, S., Gutierrez, L. K., Alfonso-Almazan, J. M., Sanz-Estebanez, S., Cordero-Grande, L., Quintanilla, J. G., Sanchez-Gonzalez, J., Marina-Breysse, M., Galan-Arriola, C., Enriquez-Vazquez, D., Torres, C., Pizarro, G., Ibanez, B., Peinado, R., Luis Merino, J., Perez-Villacastin, J., Jalife, J., Lopez-Yunta, M., Vazquez, M., … Filgueiras-Rama, D. (2021). Time-efficient three-dimensional transmural scar assessment provides relevant substrate characterization for ventricular tachycardia features and long-term recurrences in ischemic cardiomyopathy. Scientific Reports, 11(1), 18722. https://doi.org/10.1038/s41598-021-97399-w
Science
A rapid mechanism for muscle self-repair independent of stem cells
A rapid mechanism for muscle self-repair independent of stem cells
Muscle is known to regenerate through a complex process that involves several steps and relies on stem cells. Now, a new study led by researchers at Universitat Pompeu Fabra (UPF), the CNIC, CIBER-NED, and the Instituto de Medicina Molecular João Lobo Antunes (iMM, Portugal) describes a new mechanism for muscle repair after physiological damage that relies on the rearrangement of muscle fiber nuclei and is independent of muscle stem cells. This protective mechanism paves the way to a broader understanding of muscle repair in physiology and disease.
Skeletal muscle, the organ responsible for locomotion, is formed of cells (fibers) that have more than one nucleus, an almost unique feature in the body. Despite the plasticity of these fibers, their contraction can be associated with muscle damage. William Roman, first author of the study and researcher at UPF, explained: “Even in physiological conditions, regeneration is vital for muscle to endure the mechanical stress of contraction, which often leads to cellular damage. Although muscle regeneration has been investigated in depth in recent decades, most studies centered on mechanisms involving several cells, including muscle stem cells, which are required when extensive muscle damage occurs.”
“In this study, we found an alternative mechanism of muscle tissue repair that is muscle-fiber autonomous,” said study leader Pura Muñoz-Cánoves, ICREA professor and principal investigator at UPF and the CNIC.
Using in vitro models of injury and models of exercise in mice and humans, the researchers—Antonio Serrano (UPF) and Mari Carmen Gómez-Cabrera (Universitat de València e INCLIVA)—found that nuclei are attracted to sites of muscle injury, accelerating the repair of the contractile units. The team then went on to dissect the molecular mechanism underlying this phenomenon. “Our laboratory experiments with muscle cells showed that the movement of nuclei to injury sites resulted in the local delivery of mRNA molecules. These mRNA molecules are translated into proteins at injury site that act as building blocks for muscle repair,” explained William Roman. “This muscle fiber self-repair process occurs rapidly in mice and humans after exercise-induced muscle injury, and thus represents a time- and energy-efficient protective mechanism for the repair of minor lesions,” noted Pura Muñoz-Cánoves.
In addition to the implications of the study for muscle research, the movement of nuclei to injury sites introduces a concept of more general cell biological interest. “One of the most fascinating things about these cells is the movement during the development of their nuclei, the biggest organelles inside the cell, but the reasons why nuclei move are largely unknown. Now, we have shown the functional importance of this phenomenon in adulthood during cell repair and regeneration,” said study co-leader Edgar R. Gomes, group leader at the Instituto de Medicina Molecular and professor at the University of Lisbon Faculty of Medicine.
On the importance of these discoveries, Pura Muñoz-Cánoves, Antonio Serrano and Mari Carmen Gómez-Cabrera agree that “These findings constitute an important advance in the understanding of muscle biology, exercise physiology, and muscle dysfunction.”
The study was conducted at the UPF, CNIC, and CIBER-NED and at the iMM in collaboration with the University of Valencia e INCLIVA. This study was funded by the European Research Council, Association Française contre les Myopathies, the European Molecular Biology Organization, the Human Frontiers Science Program, and the Spanish Ministry of Science.
Roman, W., Pinheiro, H., Pimentel, M. R., Segales, J., Oliveira, L. M., Garcia-Dominguez, E., Gomez-Cabrera, M. C., Serrano, A. L., Gomes, E. R., & Munoz-Canoves, P. (2021). Muscle repair after physiological damage relies on nuclear migration for cellular reconstruction. Science, 374(6565), 355-+. https://doi.org/10.1126/science.abe5620
CIRCULATION
It’s never too late to treat progeria
It’s never too late to treat progeria
Scientists led by Dr. Vicente Andrés at the CNIC and the Spanish Cardiovascular Research Network (CIBER-CV) have generated the HGPSrev experimental mouse model. This is the first animal model to develop Hutchinson-Gilford Progeria Syndrome (HGPS) and also allow its suppression through the controlled regulation of the expression of progerin, the aberrant protein that causes the disease. Using the new model, the researchers have demonstrated that it is never too late to treat HGPS.
The study, published in Circulation, also establishes that the cardiovascular alterations and early death associated with HGPS can be prevented with treatments specifically targeting cells of the cardiovascular system.
HGPS is an ultra-rare genetic disease that affects fewer than 400 children worldwide and for which there is no known cure. The disease is caused by a mutation in the LMNA gene and is characterized by accelerated aging and death in the second decade of life, principally due to cardiovascular complications derived from atherosclerosis.
In the absence of mutations, LMNA encodes type A lamin proteins (Lamins A and C). The mutation found in HGPS patients results in the synthesis of progerin, a mutant protein that provokes multiple molecular and cellular alterations in the tissues where it accumulates, causing their life to pass at a highly accelerated rate, where minutes are hours and hours are lost days.
Now, thanks to the HGPSrev mouse generated by the CNIC Molecular and Genetic Cardiovascular Pathophysiology group, the research team has managed to suppress progerin expression and reestablish lamin A expression in mice of different ages, both throughout all body tissues and in specific cell types.
The characterization of the animal model was carried out with the participation of researchers at Queen Mary University of London.
Joint first authors Drs Amanda Sánchez López and Carla Espinós Estévez explained that while some palliative therapies are effective in animal models and are the subject of clinical trials, their therapeutic benefit is very limited. “A true cure would require the elimination of the culprit mutation,” commented Dr Sánchez López. However, this is not yet possible, and progeria is only diagnosed once the first symptoms have already appeared. “We therefore sought to reverse symptoms once they are already present and to determine how long treatment could be delayed and still have a beneficial impact,” explained Carla Espinós Estévez.
The extent to which the damage caused by progerin can be reversed is currently not known, and patients often do not start to receive treatment until symptoms are quite advanced. The investigators therefore addressed a key question: Can the progression of HGPS be stopped or slowed if treatment commences when the disease is advanced, or does therapeutic benefit depend on starting treatment early, when symptoms are mild?
Another important question is how treatment should be targeted. Progerin is expressed in many tissues, but it was not known if treatment needs to be directed at all affected cells or if it would be effective if targeted at a specific cell type.
To answer these questions, Dr Andrés’s team used CRISPR-Cas9 technology to generate HGPSrev mice.
The results show that HGPSrev mice show the major features of the human disease, including growth retardation, lipodystrophy, cardiovascular alterations, and early death.
The investigators also showed that elimination of progerin and restoration of lamin A expression increased life expectancy by 84.5% in HGPSrev mice with very mild symptoms; moreover, this approach extended lifespan by 6.7% even in mice with very advanced symptoms.
These results establish not only that starting treatment when symptoms are mild has a huge positive impact, but also that treatment can be beneficial no matter how late it is started.
“We managed to prevent vascular alterations and normalize survival in progeric mice by eliminating progerin expression and restoring lamin A expression specifically in vascular smooth cells and cardiomyocytes, even though other cell types remained diseased,” explained Dr Andrés.
The investigators conclude that these results “could contribute to the design of future clinical treatments, given that they suggest that strategies exclusively targeting the cardiovascular system could have a very significantly beneficial effect on patients’ life quality and expectancy.”
This study received funding from the Ministerio de Ciencia e Innovación (MCIN)/Agencia Estatal de Investigación (AEI)/10.13039/501100011033 (grants SAF2016-79490-R, PID2019-108489RB-I00, SVP-2014-068334, FJCI-2017-33299); Instituto de Salud Carlos III (ISCIII; grant AC17/00067-TREAT-HGPS), an E-Rare Joint Transnational call project, European Union Horizon 2020 Framework Programme 2017); the Fondo Europeo de Desarrollo Regional (“a way to build Europe”); the Welcome Trust (grant 098291/Z/12/Z); the Comunidad Autónoma de Madrid (grant 2017-T1/BMD-5247); the Asociación Apadrina la Ciencia-Ford España-Ford Motor Company Fund; and the Fundación “la Caixa”.
Sanchez-Lopez, A., Espinos-Estevez, C., Gonzalez-Gomez, C., Gonzalo, P., Andres-Manzano, M. J., Fanjul, V., Riquelme-Borja, R., Hamczyk, M. R., Macias, A., Del Campo, L., Camafeita, E., Vazquez, J., Barkaway, A., Rolas, L., Nourshargh, S., Dorado, B., Benedicto, I., & Andres, V. (2021). Cardiovascular Progerin Suppression and Lamin A Restoration Rescue Hutchinson-Gilford Progeria Syndrome. Circulation, 144(22), 1777-1794. https://doi.org/10.1161/CIRCULATIONAHA.121.055313
PLoS Biology
CNIC scientists identify two proteins essential for postnatal cardiac metabolism
CNIC scientists identify two proteins essential for postnatal cardiac metabolism
A study, published in PLoS Biology, shows for the first time that cardiac metabolism in the postnatal period determines the regulation of metabolism throughout the body.
Scientists at the CNIC have identified essential roles for two proteins is cardiac metabolism after birth. The study, shows that forced premature activation of these proteins in the mouse heart during the first days of life causes irreversible damage and alters whole-body metabolism, leading to diabetes and impaired thermoregulation later in life.
Lead investigator Dr. Guadalupe Sabio explained that, fortunately, the study shows that “these effects could in principle be treated through a change in diet.”
During fetal development and the first days after birth, the heart derives most of its energy from the metabolism of glucose, stored in the form of glycogen. But, explained first author Ayelén Santamans, soon after birth, the rapid growth of the heart increases the organ’s energy demands, requiring the heart to become “much more efficient at obtaining energy.”
The PLoS Biology study shows that the proteins p38γ and p38δ are activated in the heart shortly after birth and reduce the activity of the enzyme responsible for glycogen synthesis. This precipitates a metabolic change in the heart, which begins to use fatty acids as its main energy source.
Perturbation of cardiac metabolism by forced premature p38γ and p38δ expression during the postnatal period causes irreversible damage that manifests in adulthood as insulin resistance, glucose intolerance, and an impaired ability to maintain body temperature.
But the new study shows that, since the problem is an insufficient supply of energy to the heart, the damage can be repaired by a change in diet.
To demonstrate this, the researchers fed female mice a high-fat diet during pregnancy and lactation.
Newborn mice in these experiments had no cardiac damage and did not go on to develop diabetes symptoms even when p38γ and p38δ expression was prematurely activated.
The study is the first to show that heart metabolism in the postnatal period determines whole-body metabolism. “Our results show that the gradual increase in p38γ and p38δ activity is tightly regulated and that premature activation produces an energy deficit that is deleterious both for the heart and for the metabolism of the rest of the body,” said Dr Sabio.
The scientists think that both p38γ and p38δ might underlie some congenital cardiometabolic diseases that currently have no known cause, suggesting that dietary supplementation could be a valid treatment for these conditions.
The study was supported by the following funding bodies: EFSD, Lilly European Diabetes Research Programme; Ministerio de Ciencia, Innovación y Universidades; Comunidad de Madrid; Fundación Jesús Serra; Instituto de Salud Carlos III, y Fundación “la Caixa”.
Santamans, A. M., Montalvo-Romeral, V., Mora, A., Lopez, J. A., Gonzalez-Romero, F., Jimenez-Blasco, D., Rodriguez, E., Pintor-Chocano, A., Casanueva-Benitez, C., Acin-Perez, R., Leiva-Vega, L., Duran, J., Guinovart, J. J., Jimenez-Borreguero, J., Enriquez, J. A., Villlalba-Orero, M., Bolanos, J. P., Aspichueta, P., Vazquez, J., … Sabio, G. (2021). P38 gamma and p38 delta regulate postnatal cardiac metabolism through glycogen synthase 1. Plos Biology, 19(11), e3001447. https://doi.org/10.1371/journal.pbio.3001447
Science Advances
CNIC scientists identify the essential role of a gene in placental development
CNIC scientists identify the essential role of a gene in placental development
Scientists working with an experimental mouse model at the CNIC have identified the essential role of the gene GPR126 in the development of the placenta during pregnancy.
The results, published in Science Advances, show that GPR126 (adhesion G protein-coupled receptor 126) is essential for the development of a specific placental cell type that regulates the remodeling of the uterine vasculature. Cardiac defects in mouse Gpr126 mutants are secondary to placental defects, reflecting the intimate relationship between the placenta and the fetal heart.
There is evidence that GPR126 may play a similar role in placental development in humans. The babies of women who carry mutations in GPR126 die during gestation or soon after birth, and 30% of these women develop preeclampsia. This pregnancy complication, which affects 5%-8% of pregnancies in the general population, is characterized by high blood pressure that damages both mother and fetus and can result in fetal death.
Experiments in animal models have shown that GPR126 is required for the maturation of the peripheral nervous system (PNS), the formation of bone and cartilage, and the development of the inner ear. In humans, mutations in GPR126 are associated with skeletal malformations and muscle cramping in the limbs.
The CNIC Intercellular Signaling in Cardiovascular Development and Disease group, led by Dr. José Luis de la Pompa, initially identified GPR126 as a gene regulated by the NOTCH signaling pathway (a highly conserved intercellular signaling system in animals) during heart development.
This suggested that GPR126 might influence the proliferation and differentiation of cardiomyocytes (heart muscle cells) in the developing heart.
Other groups had already proposed a requirement for GPR126 in heart development in mice and zebrafish, but research had not provided conclusive evidence to confirm this hypothesis.
In the new study, the CNIC team used genetic techniques to demonstrate that GPR126 is not necessary for heart development in the mouse but plays an essential role in the formation of the placenta, a process known as placentation.
Explaining the results, Dr. de la Pompa said that “We observed that whole-body inactivation of GPR126 in mice caused thinning of the walls of the heart and resulted in embryonic death. However, when we inactivated the gene specifically in the heart, embryonic development was unaffected and heart function unaltered.”
The investigators also found that the lethal effect of whole-body GPR126 deletion “was not reversed by specific re-expression of GPR126 in the heart, indicating that embryonic death in the mutants was not due to a defect in cardiac development,” explained first author Rebeca Torregrosa.
Further studies in the zebrafish model confirmed that GPR126 “is not involved in heart development".
During embryonic development, GPR126 is expressed in giant trophoblast cells, a cell type specific to the placenta. “These cells,” noted de la Pompa, “are vitally important for the implantation of the embryo and the maintenance of pregnancy.”
The research team demonstrated that GPR126 inactivation in the embryo is compatible with survival if the placenta retains at least one normal copy of the GPR126 gene. However, inactivation of the gene in both the embryo and the placenta causes embryonic death
“One of the crucial steps in placental development is the remodeling of the maternal arteries. Known as the spiral arteries, these vessels increase their diameter to increase blood flow to the embryo. Defects in this process are associated with pregnancy complications such as preeclampsia, restricted fetal growth, and even miscarriage,” said de la Pompa.
The study shows that trophoblast GPR126 is necessary for the expression of specific proteases involved in spiral artery remodeling, which is essential for the viability of the fetus.
Based on these results, the investigators herald mice lacking GPR126 as an experimental model for studying spiral artery remodeling and preeclampsia. This animal model, moreover, has possible clinical applications in preimplantation genetic diagnosis.
The study was funded by the CIBER CV cardiovascular research network CIBER-CV (PID2019-104776RB-I00, CB16 / 11/00399), TERCEL (RD16 / 0011/0021) del Ministerio de Ciencia de España, Innovación y Universidades (MCIU), the BBVA Foundation, “La Caixa” Foundation, an EMBO fellowship, Boehringer Ingelheim Fonds, and the Programa de Atracción de Talento de la Comunidad de Madrid (Ref.2016T1 / BMD1540).
Torregrosa-Carrión R, Piñeiro-Sabarís R, Siguero-Álvarez M, Grego-Bessa J, Luna-Zurita L, Fernandes VS, MacGrogan D, Stainier DYR, de la Pompa JL. (2021). Adhesion G protein-coupled receptor Gpr126/Adgrg6 is essential for placental development; Sci Adv. 7(46):eabj5445. doi: 10.1126/sciadv.abj5445.