Research at the Center
The CNIC is organized into two departments, one focused on Basic Research and the other on Clinical Research. Research in these fields is fully interconnected through three multidisciplinary Research Areas.
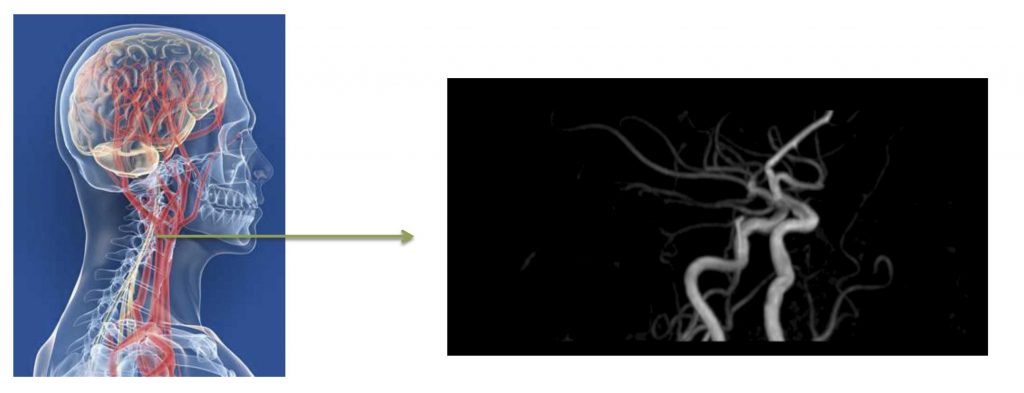
Vascular pathophysiology
Coordinator: Almudena Ramiro
The Vascular Pathophysiology Area (VPA) explores the biology of the vascular system in health and disease through multidisciplinary approaches that include molecular and cell biology, animal models of disease, and translational and clinical approaches. Our research covers the molecular mechanisms that regulate cardiovascular development and disease as well as the cellular and molecular events driving muscle regeneration and growth in health, disease, and aging. We are also interested in understanding the processes through which the organism generates new vasculature and the regulatory pathways involved in vascular wall remodeling. The VPA groups maintain an interest in atherosclerosis, the major cause of myocardial infarction and stroke. We are interested in understanding how atherosclerosis develops and the contribution of aging to these events. A central strand of this work is the exploration of early diagnosis methods and new therapeutic avenues in innovative animal models. New diagnostic and prognostic avenues are being developed through the application of state-of-the-art imaging technologies and deep proteomic analysis in population studies of subclinical atherosclerosis. A further area of interest is the immune and inflammatory component of cardiovascular disease; work in this area focuses on the role of the antibody immune response in atherosclerosis, the mechanisms of intercellular communication between immune cells, and the role of T cell and inflammatory responses in myocarditis. The Vascular Pathophysiology Area hosts three technical units: Genomics, Proteomics/ Metabolomics, and Bioinformatics, which provide state-of-the-art technology to CNIC scientists and actively contribute to the VPA research projects.
RESEARCH GROUPS
TECHNICAL UNITS
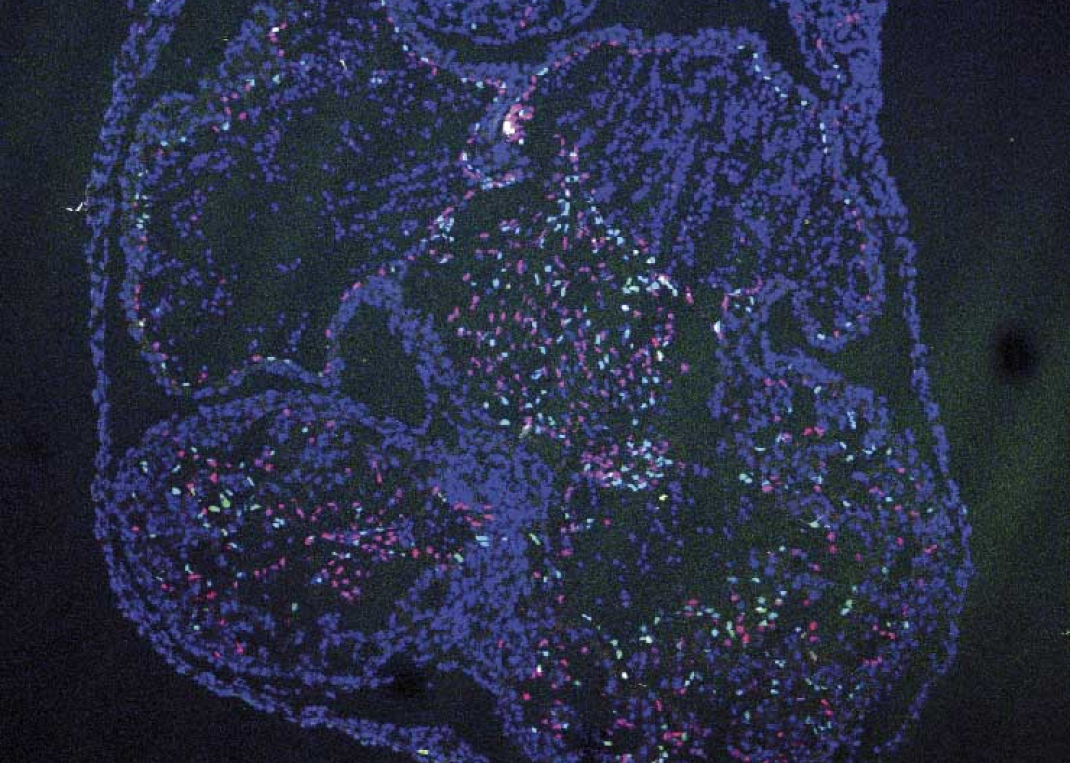
Cell and Developmental Biology
Coordinator: Miguel Ángel del Pozo
The Cell and Developmental Biology (CDB) Area comprises eight research groups and three technical units devoted to basic studies and their translational projection in the areas of vascular development, homeostasis, and disease. One research strand seeks to understand how the temporal and spatial regulation of genome architecture, transcriptional networks, and downstream functional programs determines cell fate decisions in the early embryo and different stages of heart development. These studies have potential application in the advance of regenerative medicine. Other groups investigate cell and tissue mechanisms determining cardiovascular homeostasis and disease, such as angiogenesis, inflammation, and repair. Several research lines aim to elucidate molecular principles and signaling pathways that control the cardiovascular system’s mechanical function and adaptability. This research strand deploys multidisciplinary programs integrating cell and systems biology, biophysics, and single-molecule techniques. Efforts are specifically devoted to building bridges between basic research and cardiovascular medicine, with a focus on cardiomyopathies, atherosclerosis, and cerebrovascular disease.
The three core technical units in the CDB Area work with state-of-the-art visualization techniques, providing support and developing novel technologies covering different scales and biological processes. The Microscopy Unit offers advanced confocal, multiphoton, and superresolution imaging technology and expertise, together with specific biophysical approaches for the quantification of processes in living cells and tissues. The Cellomics Unit provides cytometry analysis and separation services (including state-of-the-art spectral cytometry), as well as a full high-content functional genomics screening platform. The Microscopy and Cellomics Units together develop tailored image analysis and data processing solutions for the resolution of subcellular and tissue architecture. The Advanced Imaging Unit is a multidisciplinary group that develops new noninvasive imaging procedures to expand knowledge of the molecular and cellular events underlying cardiovascular disease. Specific programs address cardiovascular imaging, nanomedicine and radiochemistry, and metabolomics.
RESEARCH GROUPS
TECHNICAL UNITS
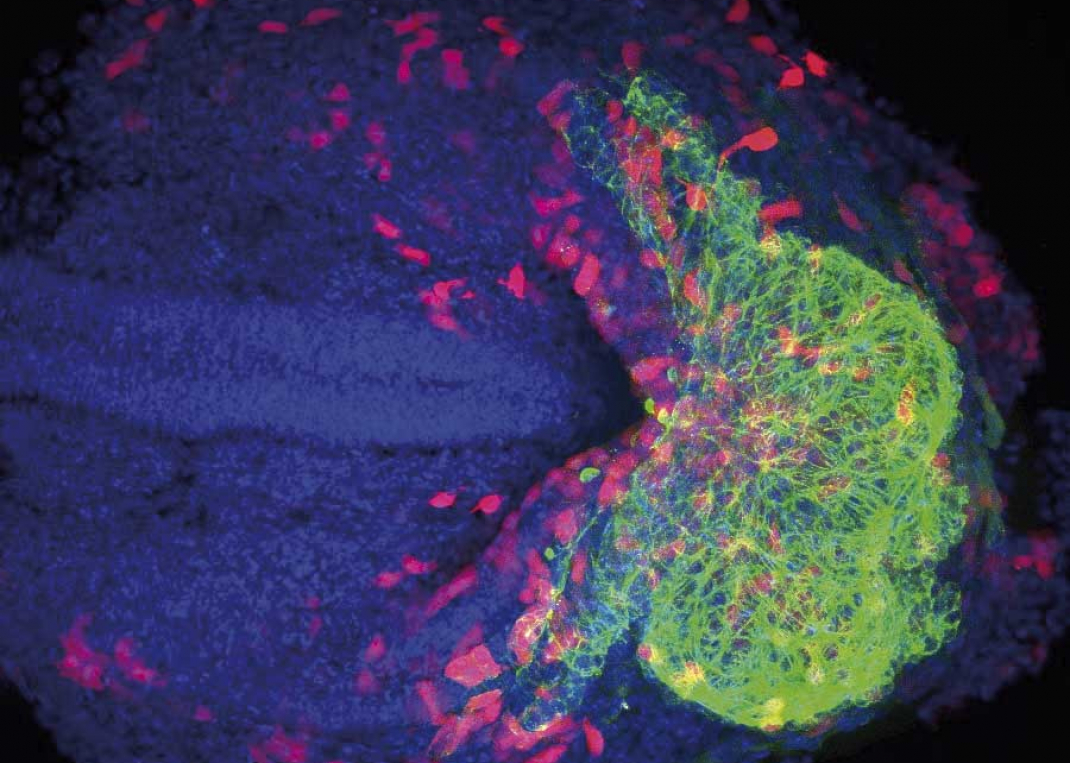
Myocardial Pathophysiology
Coordinator: David Sancho
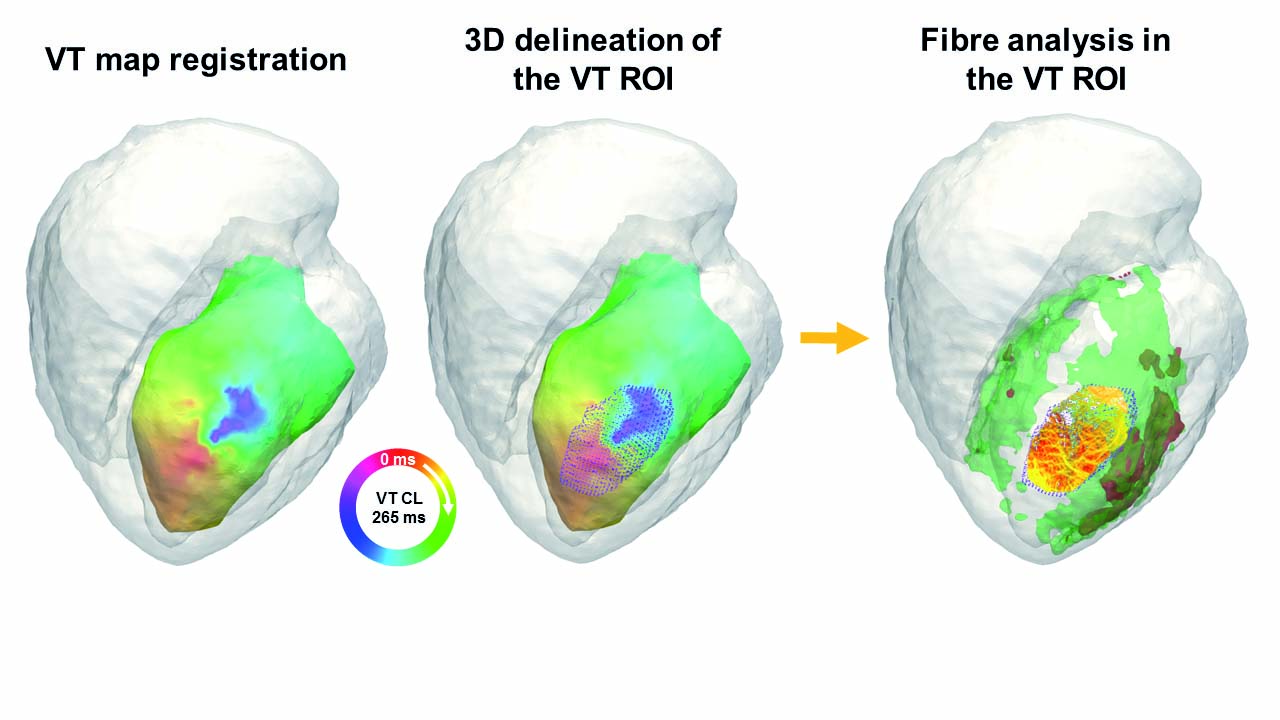
The Myocardial Pathophysiology Area (MPA) brings together scientists from multiple disciplines. Our experimental strategy comprises in vitro and in vivo studies in animal models and humans, an approach that not only provides basic understanding of health and disease, but also improves the translational potential for diagnosis and treatment. MPA groups work on several topics: the oxidative phosphorylation system, role of nuclear receptors in lipid metabolism and inflammatory responses, metabolic syndrome and stress kinases, immunobiology, inherited cardiomyopathies, cardiac arrhythmias, cardiomyocyte electrophysiology, molecular regulation of heart failure, and translational cardiovascular imaging and therapy. Our research in these fields produced several important advances in 2017: definition of a new target for the treatment of heart failure; development of new noninvasive imaging methods in mice for heart failure diagnosis; identification of the beneficial role of the serine and one-carbon metabolic pathway in the heart; description of Lafora disease as a metabolic cardiomyopathy; use of advanced magnetic resonance imaging (MRI) technology to define the postinfarction healing pattern in human hearts and identify how scar-related postinfarction cardiac fiber disorganization affects arrhythmia risk and ventricular tachycardia; investigation of the effect of protective therapies on postinfarction healing; identification of atrial necrosis as a complication of myocardial infarction contributing to chronic heart failure; description of the role of neutrophil beta 1 adrenergic receptor signaling in reperfusion injury; analysis of cardiac channels and their assembly in the cardiomyocyte; development of a proarrhythmia high content screening platform; generation of a whole transcriptome dataset for macrophages at different post-heart-injury stages; work on new strategies to manipulate immune cells for improved immunotherapy; and characterization of thermogenesis modulation in white adipose tissue during obesity and its regulation by stress kinases. Together with these research lines, MPA groups are developing noninvasive technologies through the identification of imaging, genetic, and molecular markers for the diagnosis and specific treatment of diseases that result in cardiovascular injury.
RESEARCH GROUPS
- Juan Antonio Bernal
- José Antonio Enríquez
- David Filgueiras
- Borja Ibáñez
- José Jalife
- Enrique Lara-Pezzi
- Silvia Priori
- Mercedes Ricote
- Guadalupe Sabio
- David Sancho
TECHNICAL UNITS
Selected Clinical Studies
ESSOS: a novel methodology to accelerate cardiac magnetic resonance imaging acquisition
Cardiac magnetic resonance (CMR) imaging is the gold standard for anatomical, functional, and tissue composition analysis. Wider implementation of CMR is held back by the time required to perform a complete cardiac scan, which is currently around 45 minutes. CNIC researchers and partners at Philips are working on the joint development of a revolutionary CMR sequence able to shorten the scan time to just 40 seconds. This technology has been tested in large-animal experimental models and in a pilot clinical trial. The sequence will next be tested in an MR scanner outside the CNIC, through a formal collaboration with the Instituto de Investigación Sanitaria Fundación Jiménez Díaz. The new technology will then be rolled out to scanners with varying magnetic field strengths at other collaborating hospitals.
SECURE trial
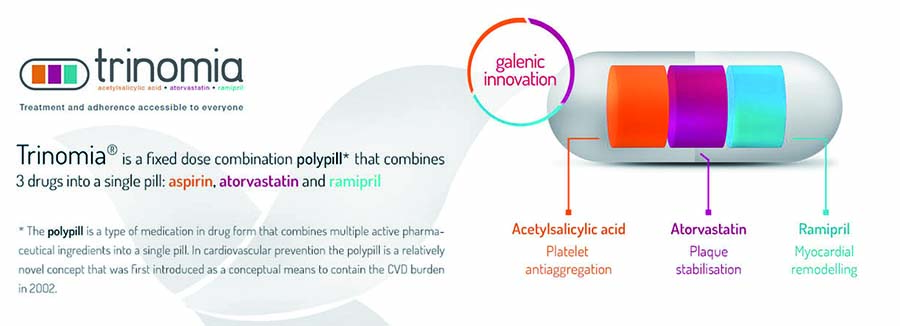
Adherence to treatment after an acute myocardial infarction (MI) is essential for efficient secondary prevention; however, a significant proportion of post-MI patients abandon prescribed therapies. CNIC researchers have developed, in partnership with Ferrer laboratories, a “polypill” that includes the three key pharmacological agents prescribed to post-MI patients (aspirin, an ACE inhibitor, and a statin). Administration of the Fuster Polypill significantly increases patient treatment adherence (J Am Coll Cardiol. 2014;64:2071-82), and CNIC researchers are now leading SECURE, a multinational randomized clinical trial supported by the EU H2020 Programme. This ongoing trial (trial identifier NCT02596126) will enroll >3000 patients soon after an MI and randomize them to standard treatment vs a Fuster Polypill-based strategy. Patients will be followed for a minimum of 2 years and the incidence of major cardiovascular events will be evaluated.
SPHERE-HF trial
Pulmonary hypertension (PH) secondary to left heart disease (group 2 PH) is the most common form of PH and currently lacks effective therapy. CNIC researchers have identified a novel therapeutic target for this disease in a large animal model of PH: the β3 adrenergic receptor (Basic Res Cardiol. 2016;111:49). The CNIC is currently leading a phase 2 clinical trial in which group 2 PH patients are randomized to standard therapy vs standard therapy plus a β3 selective agonist (trial identifier NCT02775539). A total of 80 patients are being recruited in four Spanish hospitals and will be followed under treatment for 4 months. The study endpoints are pulmonary artery hemodynamics and the CMR profile.
PESA-CNIC-Santander study
PESA-CNIC-Santander is a long-term collaboration between the CNIC and Banco Santander. This study aims to identify the presence of atherosclerosis long before symptoms appear and to understand the cues that lead to its development and progression. The study, led by CNIC General Director Valentín Fuster, was initiated in 2010 and enrolled 4200 asymptomatic participants between the ages of 40 and 55 years. Participants undergo a battery of imaging and analytical tests, including 3D vascular ultrasound detection of atherosclerotic plaques in the carotid, aorta, and iliofemoral territories, together with coronary artery calcium quantification. Participants undergo these studies every 3 years, and those showing signs of preclinical atherosclerosis are further examined with other noninvasive imaging technologies. This flagship study involves contributions from several of the CNIC’s clinical and basic research groups and has already produced several seminal publications.
H2H study
There is increasing awareness of the association between atherosclerosis and cognitive function. The mechanisms linking these processes are not fully understood. The Heart-to-Head (H2H) study is testing the hypothesis that extensive subclinical atherosclerosis is associated with subtle cognitive decline and beta-amyloid deposition in the brain. This transatlantic collaboration is framed within an agreement between the CNIC and Mount Sinai Hospital in New York, with CNIC General Director Valentín Fuster as principal investigator. In Spain, the H2H project is coordinated by the CNIC and the Hospital 12 de Octubre. Other university hospitals (Fundación Jiménez Díaz, Clínico San Carlos, and Gregorio Marañón) participate in this pioneering endeavor, which receives funding from the Carlos III Institute of Health through a Proyecto integrado de excelencia. A total of 300 participants are undergoing cognitive function testing in combination with extensive atherosclerosis phenotyping (multiterritory 3D vascular ultrasound, cardiac computed tomography) and thorough brain imaging (anatomical and functional magnetic resonance imaging and positron emission tomography (PET)-amyloid scan).