Nature: Researchers characterize rare, damaged cells that block the functions of their neighbour healthy cells and identify ways to neutralize them and improve tissue regeneration
The finding provides a basis for mitigating the loss of muscle regenerative capacity in elderly people and for improving muscle repair in young healthy people
Researchers at the Universitat Pompeu Fabra (UPF), ICREA, CIBERNED, CNIC and Altos Labs, among other national and international collaborators, have characterized how damaged cells (senescent cells) that inevitably arise after injury negatively impact tissue regeneration, and how this mechanism operates actively in old age, but surprisingly also in young age. This negative action can be overcome genetically and pharmacologically, hence restoring stem cell regenerative functions.
Tissue regeneration depends on a population of stem cells and its neighbouring cells, a process whose efficacy declines with aging. The reasons of this decline remain largely unknown.
Dr. Pura Muñoz-Cánoves, ICREA professor at the Department of Medicine and Life Sciences (MELIS) at UPF in Barcelona, CNIC in Madrid, and CIBERNED, and now in Altos Labs San Diego Institute of Science, and Dr. Eusebio Perdiguero (also from MELIS and now in Altos Labs), have found in experiments with mice that senescent cells are new regulatory components of the muscle tissue regenerating niche that blunt muscle regeneration at all stages of life.
Cellular senescence is a state of irreversible cell cycle arrest that often emerges after tissue damage and in age-related diseases. Cells don’t die but remain in a hibernation state. Together with apoptosis (a form of programmed cell death), senescence is one of the mechanisms the body uses to control the unwanted proliferation shown by tumours. Therefore, the study of these cells is of great biomedical relevance. In addition, senescent cells affect tissue repair processes, and beneficial effects as tumour suppressors have been documented during embryo development and in liver and skin repair or reprogramming.
Despite these reasons, few studies had attempted to profile and characterize them in vivo. This is largely attributed to the rarity and scarcity of these cells, even in aged tissues.
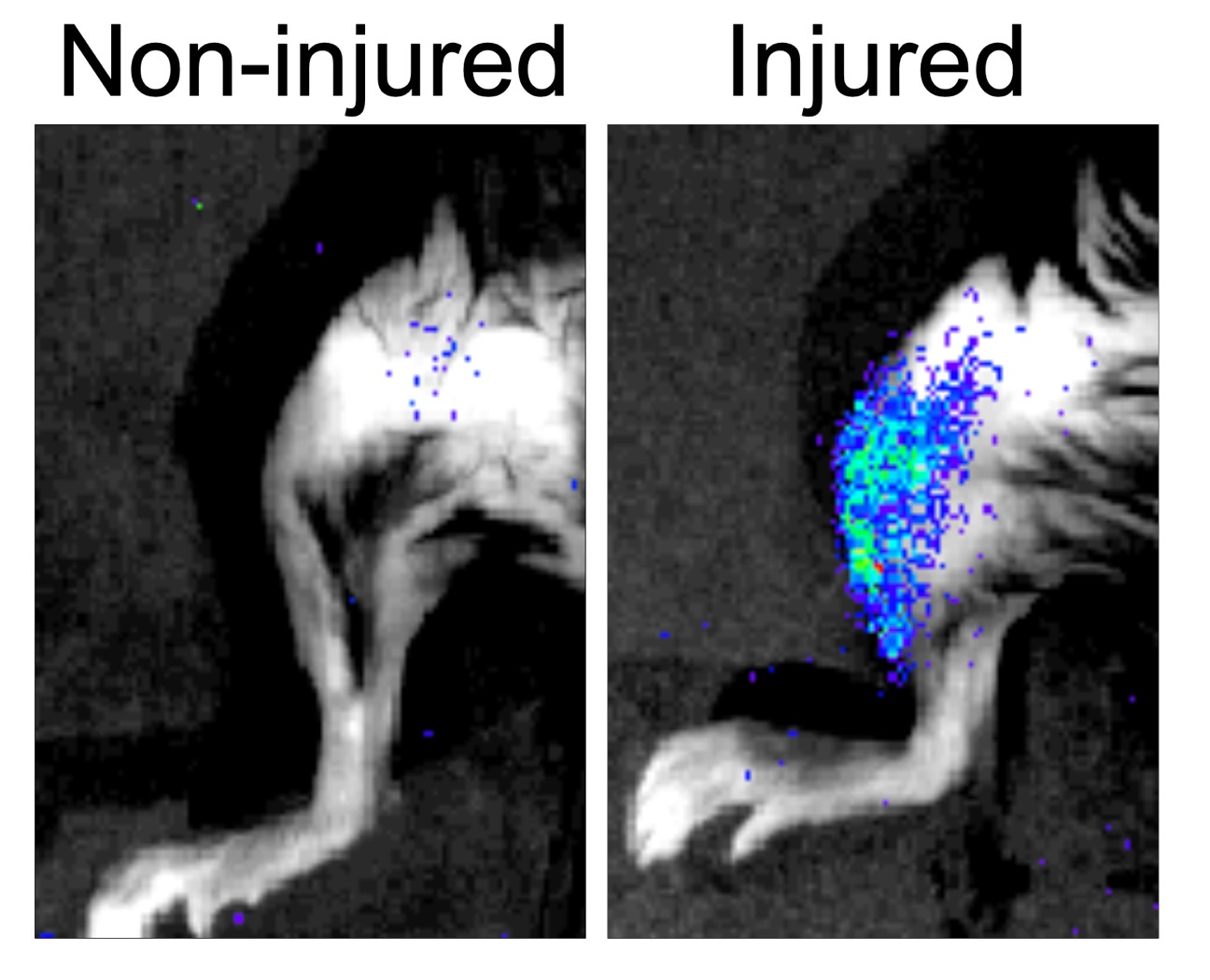
In a study published today in Nature, the team of researchers generated the first transcriptomic atlas of senescent cells of damaged skeletal muscle of mice of distinct ages (transcriptomic refers to everything related to RNA or the structures that transcript the information encoded originally inside a nucleus cell). Researchers found that senescent cells are widely heterogeneous, yet they display common traits, including the secretion of proinflammatory and profibrotic (that promotes an excess of fibrous connective tissue) factors. This secretion in turn, impacts the nearby stem cells and hampers their regenerative capacity, thus impairing muscle regeneration. So, it appears that what once was as a good protection tool now turns into a bad one.
Results showed that reducing the load of senescent cells (either through genetic or pharmacological treatments that induce death of these cells) improved the regeneration of aged muscles and, unexpectedly also, of young muscle. These benefits in young tissue are due to reduction of inflammation in the stem cell nearby environment, which fosters stem cell functions.
“This is consistent with the notion that senescent cells, even in young tissues, create a hyper inflamed microenvironment that mirrors inflammation associated to ageing (inflammaging)”, says Pura Muñoz-Cánoves. Thus, senescent cells provoke the anticipated ageing of the stem cell niche even in young mice; hence, reducing the senescent burden, attenuates inflammation of the stem cell niche and improves muscle repair.
“In addition to the biomedical benefits of targeting senescent cells, the new molecular information provided by the muscle senescent cell atlas could be likely transferred to understanding the function of senescence in other tissues whose senescent cells have either not been profiled at all or lack enough senescent cell numbers”, says Dr. Eusebio Perdiguero.
Increasing work from many groups demonstrates that the effects of senescent cells are diverse (beneficial or detrimental) and depend on the tissue environment and type, the duration of injury, the degree of persistence of senescent cells, and the organism’s age. Thus, “the roles of senescent cells should be studied in distinct contexts, in normal, aged and disease states,” indicates Dr. Muñoz-Cánoves. In this line, she adds that “altogether, the information shown in this paper will be instrumental for advancing our knowledge of senescent cells and finding new treatments to target them in the context of regenerative medicine and aging”.
The study has had the participation of the CNIC's Genomic group led by Dr. Ana Dopazo.
This scientific study has also involved the collaboration of researchers at the Kyushu University, (Fukuoka, Japan), Altos Labs San Diego Institute (San Diego, USA), University of Tokyo (Tokyo, Japan), Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences (Guangzhou, China), CIC bioGUNE (Derio, Spain), Institute for Research in Biomedicine (IRBB) and BIST (Barcelona, Spain), Luxembourg Centre for Systems Biomedicine (LCSB), University of Luxembourg (Luxembourg). The study was funded in part by grants from the European Research Council (ERC), the Spanish Ministry of Science and Innovation, La Caixa Foundation, AFM, MDA, MWRF, and DPP-Spain.
- Victoria Moiseeva et al. “Senescence atlas reveals tienen aged-like inflamed anida that blunts muscle regeneration”. Nature, 2022