Immunity: CNIC scientists uncover a novel mechanism involved in inflammation resolution
A study published in Immunity reveals that type I interferons (IFN‑I) help resolve inflammation by inducing changes in macrophage mitochondria
When our body fights an infection, the immune system must quickly activate defenses and trigger a beneficial inflammatory response. But it is just as important to resolve that inflammation and return to homeostasis. Macrophages play a key role in this balance: they are cells specialized in “phagocytosing,” or engulfing, cells that have died due to viral infection and in repairing infection ‑ or inflammation ‑ related tissue damage.
A study conducted at the Centro Nacional de Investigaciones Cardiovasculares Carlos III ( (CNIC) and published in Immunity reveals the mechanism by which a signal associated with antiviral and inflammatory responses—type I interferon (IFN‑I)—tunes macrophage mitochondria to enhance the clearance of tissue damage and prevent uncontrolled inflammation.
IFN‑I is a cytokine that can promote either inflammatory or anti‑inflammatory responses, depending on the disease context. It activates a specific inflammatory program known as interferon‑stimulated genes (ISGs). When kept in check, inflammation is beneficial: it helps macrophages “clean up” dead cells and repair tissue damage, for example during viral infections. The newly published study answers the question of how IFN‑I regulates macrophage function for inflammation resolution.
The CNIC team shows that when macrophages detect nucleic acids associated with viral infections inside the cell, they change their metabolism. Their mitochondria lower the membrane potential but continue to function properly. This change occurs through the production of IFN‑I in response to viral infection, which binds to the IFN‑I receptor on the macrophage and triggers the production of the protein ISG15.
«We found that a specific interferon‑stimulated protein, called ISG15, binds to mitochondrial proteins and provokes two coordinated changes: increased ATP production and a reduction in mitochondrial membrane potential. These metabolic changes in macrophages improve their ability to remove dead cells, which helps inflammation resolution», explains first author Gillian Dunphy, a researcher in the Immunobiology group led by David Sancho.
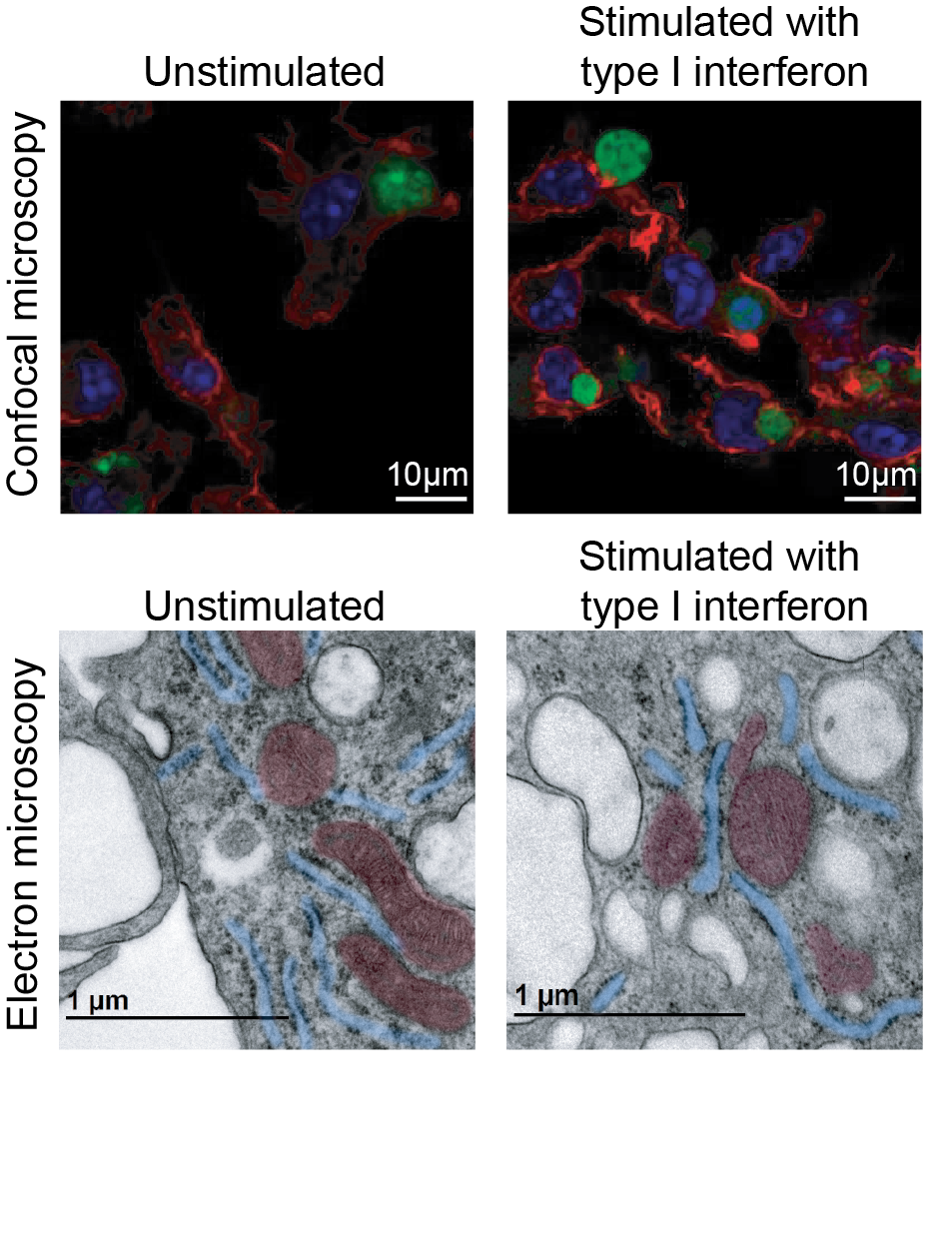
Dunphy adds: «In addition, the drop in mitochondrial membrane potential activates a protease that increases mitochondrial fragmentation. This reshapes metabolism and reduces the expression of inflammatory genes, so that IFN‑I itself promotes the resolution of inflammation».
To reach these conclusions, the researchers combined metabolic analyses, advanced microscopy, and cellular and animal models. They observed that treatment with IFN‑I increases macrophage uptake of apoptotic cells and that this improvement disappears in the absence of ISG15. They also found that changes in mitochondrial shape and communication act as a natural regulator that prevents excessive interferon signaling—something that, in other contexts, is associated with autoinflammation.
David Sancho, head of the CNIC Immunobiology Laboratory, explains: «This finding maps out a new way in which antiviral signals engage with cellular metabolism to balance defense and repair. Understanding and modulating this axis could inspire strategies to accelerate the resolution of inflammation in different pathologies or to fine‑tune interferon‑based treatments, maximizing benefits while minimizing undesired effects».
The study was conducted in collaboration with research teams led by Susana Guerra (Universidad Autónoma de Madrid) and Francisco Sanchez-Madrid (Hospital Universitario La Princesa, Madrid).
Work in the DS laboratory is funded by the CNIC; by Ministerio de Ciencia, Innovación y Universidades, Agencia Estatal de Investigación; Comunidad de Madrid; by Scientific Foundation of the Spanish Association Against Cancer; by Worldwide Cancer Research; by the European Union ERC; the European Molecular Biology Organization; Marie Skłodowska-Curie Actions, by CRIS Foundation against Cancer and by “la Caixa” Foundation.
- Dunphy G, Adán-Barrientos I, Fernández-Delgado I, Villarroya-Beltri C, Heras-Murillo I, Moya-Ruiz E, et al. A type I interferon–mitochondrial axis regulates efferocytosis and interferon-stimulated gene induction in macrophages. Immunity. 2026;xx(x):xxx–xxx. doi:10.1016/j.immuni.2025.12.010











