Cell: The unexpected repair function of neutrophils
CNIC scientists have discovered previously unsuspected actions of the immune system that help to maintain organ health
Scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) have discovered that neutrophils, the most abundant cells of the innate immune system, have many more functions in the body than previously thought. This finding, promoted by the “la Caixa” Foundation, opens up new therapeutic possibilities for the treatment of multiple diseases, such as cancer..
In a study published in the journal Cell, the research team demonstrate that neutrophils acquire new characteristics when they arrive in a tissue and that these specialized functions help to maintain organ health.
The cells of the immune system defend the body against external pathogens, providing protection against microorganisms that cause disease, while also helping to repair injuries such as wounds and bone fractures.
The different types of immune cells include lymphocytes and the cells of the innate immune system. “Lymphocytes produce antibodies or receptors that specifically target viruses or bacteria to build immunity against these pathogens. The cells of the innate immune system, on the other hand, provide a faster but nonspecific response that can sometimes trigger uncontrolled inflammation, as happens in the lungs of patients with severe COVID-19, for example,” explained Dr Andrés Hidalgo, lead investigator on the study.
Every day, the marrow inside our bones produces immense quantities of neutrophils. These cells then enter the bloodstream and are distributed to almost all tissues of the body. Neutrophils have a short lifespan, living for less than 24 hours. For this reason, scientists believed that these cells had a very limited capacity to adapt to their environment and adopt new functions.
But in the Cell study, “we found that when neutrophils leave the circulation and migrate into tissues they acquire new, previously unknown properties”, said Dr Hidalgo.
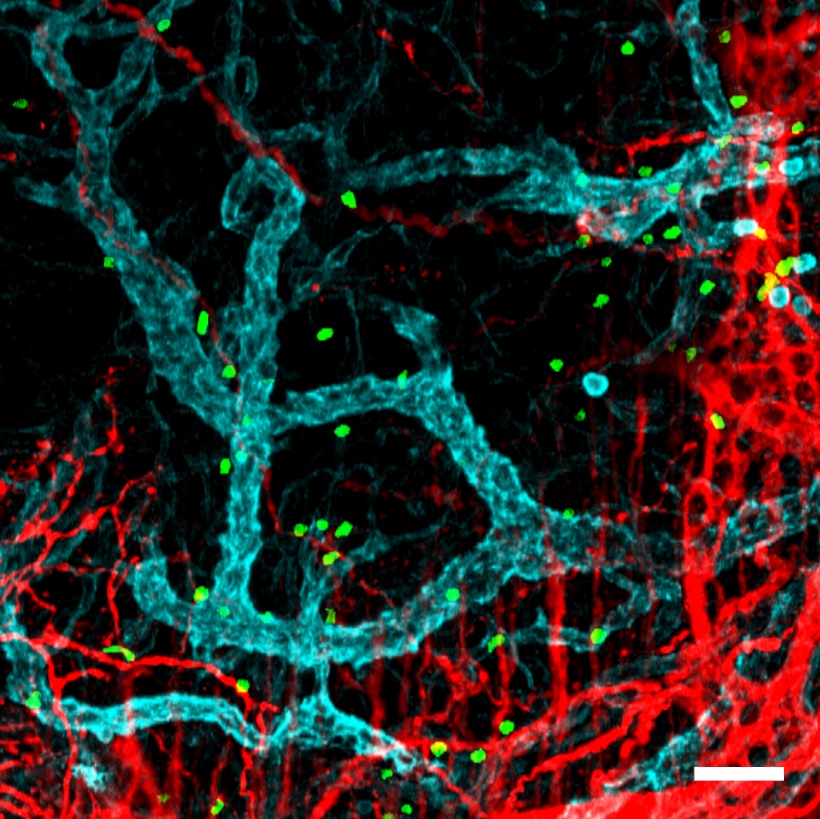
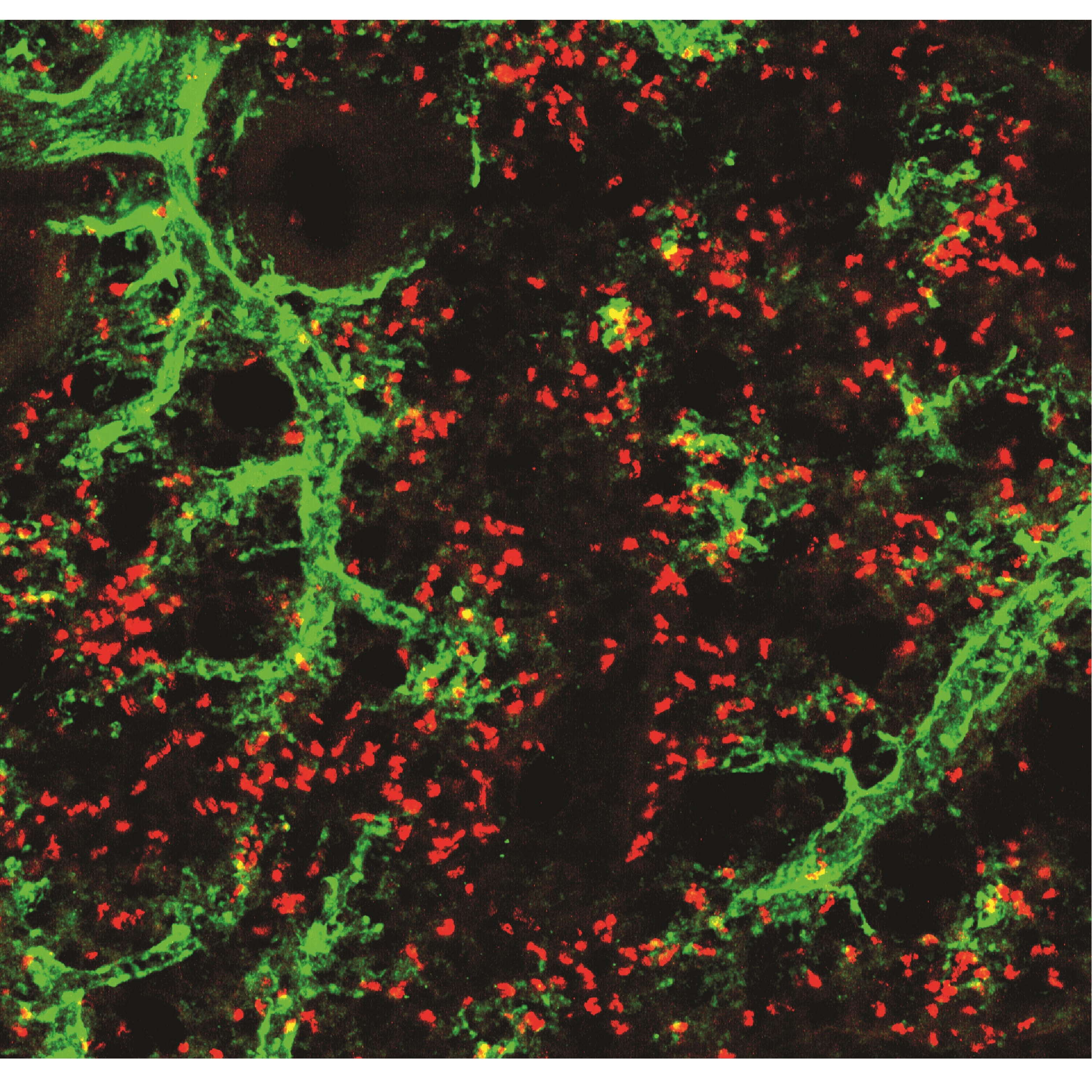
“What is fascinating is that neutrophils appear to acquire functions useful to the specific tissues in each organ. For example, we found that neutrophils in the lung acquire the ability to contribute to the formation of blood vessels, whereas neutrophils in the skin help to maintain the integrity of the cutaneous epithelium. This ability to change cell properties was identified in healthy individuals, which suggests that neutrophils participate in a great variety of normal functions in the body and are not limited to combating infection,” said Dr Hidalgo.
The identification of these adaptations allows a better understanding of the roles of different immune cells in disease
Historically, scientists have viewed the innate immune system as a collection of cells with fixed, nonspecific responses. But in recent years, some researchers have found evidence that these cells can acquire highly specific functions. According to co-lead investigator and first author Iván Ballesteros, “this is particularly exciting because if we can define the mechanisms that control how these cells acquire new functions we will be able to design new treatments to exploit this plasticity of neutrophil responses for the benefit of patients.”
In cancer, for example, tumors need to promote the generation of new blood vessels in order to grow. To block tumor growth, scientists therefore need to understand how tumors co-opt the plasticity of the immune system to promote the formation of these blood vessels. For Ballesteros, a major point of interest in the new study is that “the results show that neutrophil immune plasticity is not dependent on the presence of disease, suggesting that it has beneficial functions that sometimes get short-circuited in pathological settings.”
Previous studies had already identified neutrophil heterogeneity in several diseases. Indeed, these neutrophil changes are prognostic markers in cancer and help to regenerate blood cells after bone marrow transplantation.
The study, published in Cell, shows that the immune cells called neutrophils undergo specific changes to match the specialized needs of each organ in the body
However, the mechanisms that establish neutrophil hyperplasticity are poorly understood, and the new results are a crucial step towards filling this knowledge gap. “Essentially, what we have demonstrated is that neutrophils, despite their sort lifespan, can change their function and that they do this when they enter tissues. The identification of these adaptations allows a better understanding of the roles of different immune cells in disease,” explained Andrea Rubio, joint first author on the study and a bioinformatician at the CNIC.
This study was funded by the European Regional Development Fund (ERDF), RTI2018-095497-B-I00, the “la Caixa” Foundation, and the Transatlantic Network of Excellence (TNE-18CVD04) of the Leducq Foundation.











