Frontiers Immunology: CNIC scientists show that trained innate immunity protects against SARS-CoV-2 and increases the immunogenicity of COVID-19 vaccines
Immune therapy based on nasal administration of a preparation of dead bacteria called MV130 prevents death in experimental animals infected with the SARS-CoV-2 coronavirus and increases the immunogenicity of COVID-19 vaccines
COVID-19 vaccines provide the best weapon against the pandemic caused by the SARS-CoV-2 coronavirus. But these vaccines have also highlighted the need for effective and fast acting responses against future viral threats. This can be achieved by activating–or training–a part of the body’s natural defense system called innate immunity. This is the conclusion of a multicenter study coordinated by Spanish scientists Carlos del Fresno, of the Instituto de Investigación Sanitaria del Hospital Universitario La Paz (IdiPAZ), Juan García Arriaza and Mariano Esteban of the Centro Nacional de Biotecnología (CNB)/Consejo Superior de Investigaciones Científicas (CSIC), and David Sancho of the Centro Nacional de Investigaciones Cardiovasculares (CNIC).
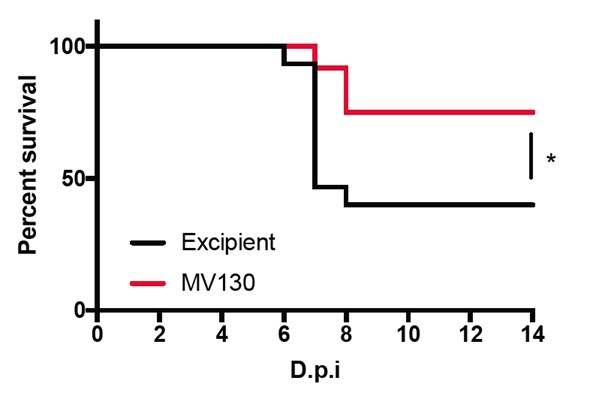
In the study, published today in Frontiers in Immunology, the Spanish team demonstrate that immune therapy with the MV130 dead bacterial preparation, produced by the Spanish biotech CompanyInmunotek S.L. in Alcalá de Henares, protects against SARS-CoV-2 infection in susceptible mice. The study was carried out at the Centro de Investigación en Sanidad Animal (CISA), part of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA-CSIC), in Madrid. The mortality rate after SARS-CoV-2 infection was significantly lower in mice that had previously received the MV130 immune therapy. This treatment trains the innate immune system by introducing epigenetic changes into innate immune cells, as demonstrated in previous studies in Dr. Sancho’s laboratory.
COVID-19 vaccines protect against infection with SARS-CoV-2 by exposing the body to viral antigens to induce a type of immune memory. Until a few years ago, scientists believed that the only form of immune memory was specific immunity of this type, called adaptive immunity. This is the body’s ability to ‘remember’ previous encounters with pathogens such as viruses and bacteria and to trigger a protective immune response upon re-exposure to the same pathogen components. In contrast, innate immunity, which is not specific to a particular pathogen, was believed not to be endowed with memory. However, “today we know that the innate immune system can be trained to give an improved response to unrelated future infections. For example, training the innate immune system with bacterial preparations can protect against infection with viruses such as SARS-CoV-2. Moreover, the effects of this training are long-lasting,” said David Sancho.
The research team investigated whether this immune therapy might improve the immune response generated in response to subsequent vaccination against COVID-19. In experiments carried out at the CNIC, mice treated with MV130 or a control preparation were innoculated with one of two types of vaccine: one based on the CSIC MVA-CoV2-S vaccine (generated by Drs. García Arriaza and Esteban) and another based on recombinant protein S with adjuvant. Two vaccine administration routes were tested: intramuscular injection and the intranasal route
“Animals treated with MV130 before vaccination, which therefore had a trained innate immune system, had a better immune responses to vaccination,” explained Carlos del Fresno. “MV130 treatment increased the activation of cytotoxic CD8 T cells, which eliminate infected cells, as well as the levels of IgA-class antibodies to SARS-CoV-2 protein S in mucous membranes.”
Protection against death
Summarizing the impact of the findings, Juan García Arriaza said: “The study shows that immune therapy with MV130 directly protects against death from SARS-CoV-2 infection and shows the ability of trained innate immunity to improve immune responses generated by COVID-19 vaccines.”
Mariano Esteban continued: “These important results indicate that immune therapy with MV130 could offer valuable protection to vulnerable groups threatened by infection with emerging pathogens until specific vaccines become available.”
The authors conclude that MV130 immune therapy will improve vaccine efficacy and immunogenicity, especially in vulnerable population groups or against variants against which vaccines are less effective, thus contributing to better community protection against COVID-19.
The study was supported by funding from the Fondo Solidario Juntos Banco Santander for Dr. David Sancho’s research at the CNIC and the Fondo COVID-19 Instituto de Salud Carlos III for Dr. Juan García Arriaza’s work. Other supporting bodies were the Fundación Asociación Española contra el Cáncer (AECC); Fondo Supera COVID-19 (Crue Universidades-Banco Santander); Fundación la ‘Caixa’; Programa Horizonte 2020 y Beca Marie Skłodowska-Curie de la Unión Europea; an EMBO fellowship; Comunidad de Madrid y Fondo Social Europeo y Desarrollo Regional Europeo; Consejo de Investigación Europea (ERC-2016-Consolidator Grant 725091); Agencia Estatal de Investigación (PID2019-108157RB); Comunidad de Madrid; Inmunotek S.L ., y Fundació La Marató de TV3.











