Nature Cardiovascular Research: Mutations acquired by blood cells are an indicator of cardiovascular risk
Researchers from the CNIC and Columbia University (USA) review the role of acquired mutations and clonal hematopoiesis in cardiovascular disease
Scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) in Madrid, Spain and Columbia University, New York have published a review article in Nature Cardiovascular Research examining the role in cardiovascular disease of acquired mutations linked to clonal hematopoiesis.
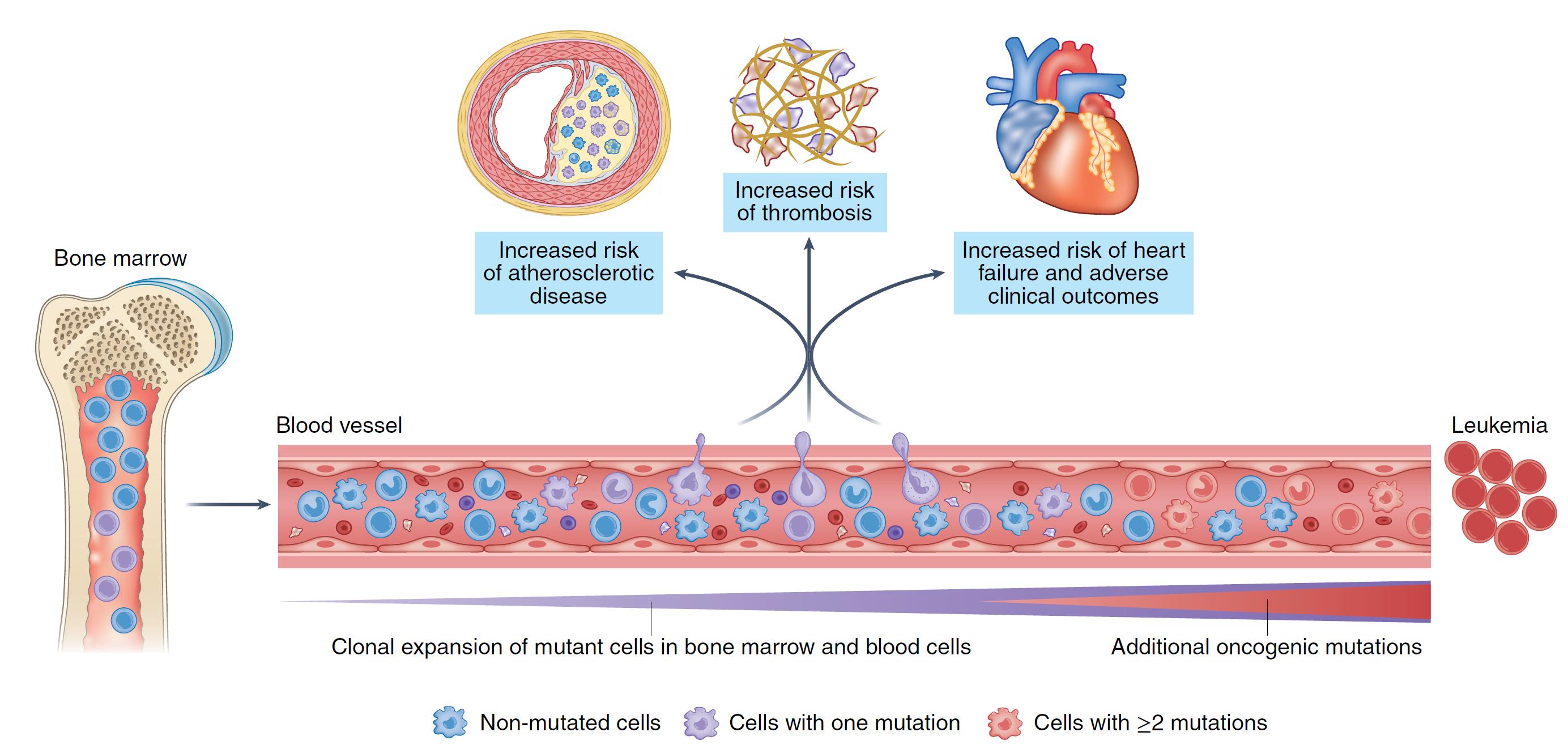
The human body generates thousands of millions of blood cells every day, and this phenomenal production unavoidably entails random replication errors in the genetic material: DNA mutations acquired by progenitor cells are transmitted to their progeny, including cells of the immune system. Most of these mutations are harmless, but some of them confer a competitive advantage on their host cells, resulting in their progressive expansion to form blood cell clones. This phenomenon is known as clonal hematopoiesis, and several recent lines of evidence suggest that it might act as an indicator of cardiovascular risk, independently of traditional factors.
In an article published in Nature Cardiovascular Research, CNIC scientist José Javier Fuster and Dr. Alan Tall of Columbia University review current knowledge on the role of clonal hematopoiesis in cardiovascular disease, highlighting areas where further research promises to transfer recent advances into tools for the prevention or treatment of cardiovascular disease.
The review article, the authors dissect the various mechanisms underlying this increased inflammation
Numerous clinical studies in recent years have demonstrated that acquired mutations linked to clonal hematopoiesis are associated with an elevated risk of multiple cardiovascular diseases, including myocardial infarction, stroke, and heart failure. In recent years, cardiovascular researchers have debated the possibility that these mutations, which are also linked to a heightened risk of hematological cancer, contribute directly to the development of disease.
Work in several laboratories, including those led by Drs. Fuster and Tall, has shown that some mutations linked to clonal hematopoiesis accelerate the development of cardiovascular disease and aggravate inflammatory responses.
“A deeper understanding of these mechanisms could, in the future, lead to personalized strategies for cardiovascular prevention, aimed specifically at countering the proinflammatory effects of these mutations,” said José Javier Fuster.
Some of these possible strategies are discussed in the review article.
Despite the availability of effective treatments for traditional cardiovascular risk factors, cardiovascular disease continues to be the leading cause of death, explained Dr. Fuster.
Scientists have known for some years that inflammation is a central factor in cardiovascular disease, but this has not led to the general use of anti-inflammatory treatments for cardiovascular conditions.
These treatments should only be used in specific situations, since they can increase the risk of infectious disease.
The authors write that “the detection of acquired mutations linked to clonal hematopoiesis could help identify those people who could benefit most from anti-inflammatory treatment, when presenting with exacerbated inflammation, and inform which specific treatment could be especially effective”.
Achieving this goal will, the authors conclude, require detailed research into the effects of different mutations associated with clonal hematopoiesis and, eventually, clinical trials of candidate treatments.
Drs. Fuster and Tall are members of the Clonal Hematopoiesis and Atherosclerosis Network, a Leducq Foundation-funded association of pioneering researchers in the field that also includes CNIC scientist Andrés Hidalgo.











