PLoS Biology: CNIC scientists identify two proteins essential for postnatal cardiac metabolism
The study, published in PLoS Biology, shows for the first time that cardiac metabolism in the postnatal period determines the regulation of metabolism in the whole body
Scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) have identified essential roles for two proteins is cardiac metabolism after birth. The study, published in PLoS Biology, shows that forced premature activation of these proteins in the mouse heart during the first days of life causes irreversible damage and alters whole-body metabolism, leading to diabetes and impaired thermoregulation later in life.
Lead investigator Dra. Guadalupe Sabio explained that, fortunately, the study shows that “these effects could in principle be treated through a change in diet.”
During fetal development and the first days after birth, the heart derives most of its energy from the metabolism of glucose, stored in the form of glycogen. But first author Ayelén Santamans explained that, soon after birth, the rapid growth of the heart increases the organ’s energy demands, requiring the heart to become “much more efficient at obtaining energy.”
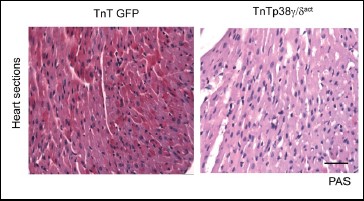
The PLoS Biology study shows that the proteins p38γ and p38d are activated in the heart shortly after birth and reduce the activity of the enzyme responsible for glycogen synthesis. This precipitates a metabolic change in the heart, which begins to use fatty acids as its main energy source.
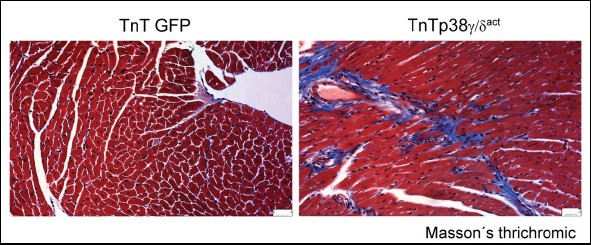
Perturbation of cardiac metabolism by forced premature p38γ and p38d expression during the postnatal period causes irreversible damage that manifests in adulthood as insulin resistance, glucose intolerance, and an impaired ability to maintain body temperature.
But the new study shows that, since the problem is an insufficient supply of energy to the heart, the damage can be repaired by a change in diet.
To demonstrate this, the researchers fed female mice a high-fat diet during pregnancy and lactation.
Newborn mice in these experiments had no cardiac damage and did not go on to develop diabetes symptoms even when p38γ and p38d expression was prematurely activated.
The study is the first to show that heart metabolism in the postnatal period determines whole-body metabolism. “Our results show that the gradual increase in p38γ and p38δ activity is tightly regulated and that premature activation produces an energy deficit that is deleterious both for the heart and for the metabolism of the rest of the body,” said Dr Sabio.
The scientists think that both p38γ and p38δ might underlie some congenital cardiometabolic diseases that currently have no known cause, suggesting that dietary supplementation could be a valid treatment for these conditions.
The study was supported by the following funding bodies: EFSD, Lilly European Diabetes Research Programme; Ministerio de Ciencia, Innovación y Universidades; Comunidad de Madrid; Fundación Jesús Serra; Instituto de Salud Carlos III, y Fundación “la Caixa”.











