Inherited Cardiomyopathies
The group is focused on the study of the genetic mechanisms involved in the development of familial heart disease and heart failure.
In recent years, the main line of research of the group has been the understanding of the mechanisms that lead to phenotype expression in familial (genetic) cardiomyopathies. It is intriguing why despite the advances in genetic field, a genetic cause is still only detected in less than 40% of the families with Hypertrophic cardiomyopathy (HCM) and Dilated cardiomyopathy (DCM), the two main types of cardiomyopathies. Therefore, the group is focused in identifying new genes and new mutations that could explain the cause of the disease in the unsolved group of patients.
Another area of interest relies in the fact that in many of the identified DCM and HCM disease-causing genes the knowledge about the disease’s clinical course is still scarce, which makes it difficult to establish a prognosis, and anticipate complications. Thanks to the broad clinical and genetic expertise and the collaboration with several international centers, the group has contributed significantly to understanding the natural history of DCM associated with mutations in several genes, determining genotype/phenotype correlations and gene specific manifestations, and establishing prognosis and response to therapy (Amor-Salamanca et al, J Am Coll Cardiol 2017, Dominguez et al, J Am Coll Cardiol 2018, Lopez-Sainz et al, J Am Coll Cardiol 2020, Restrepo-Cordoba et al, Eur J Heart Fail 2021, Escobar-Lopez et al. J Am Coll Cardiol 2021).
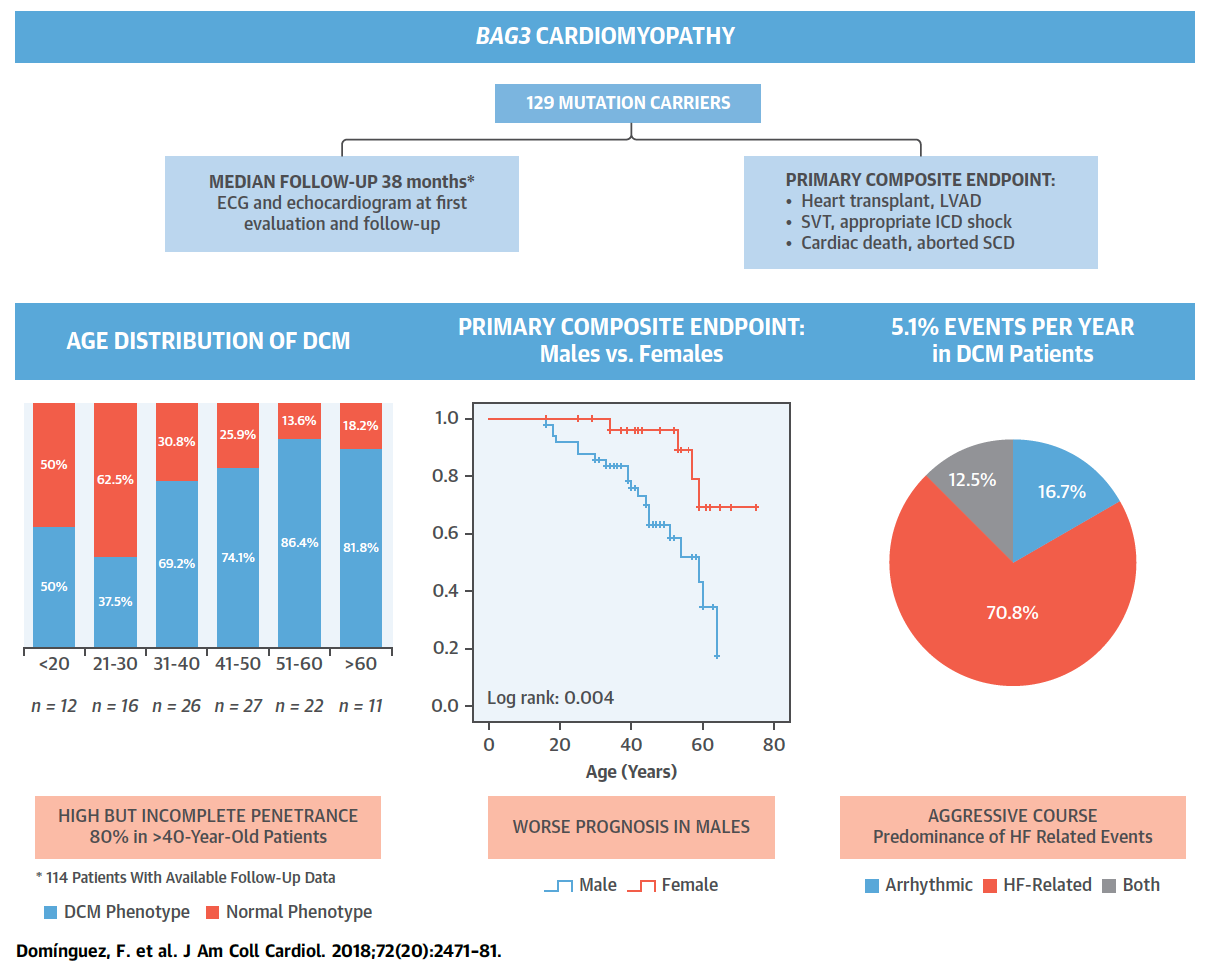
Figure 1. Clinical Outlook and Outcomes of 129 Patients With BAG3 Mutations. Full paper here.
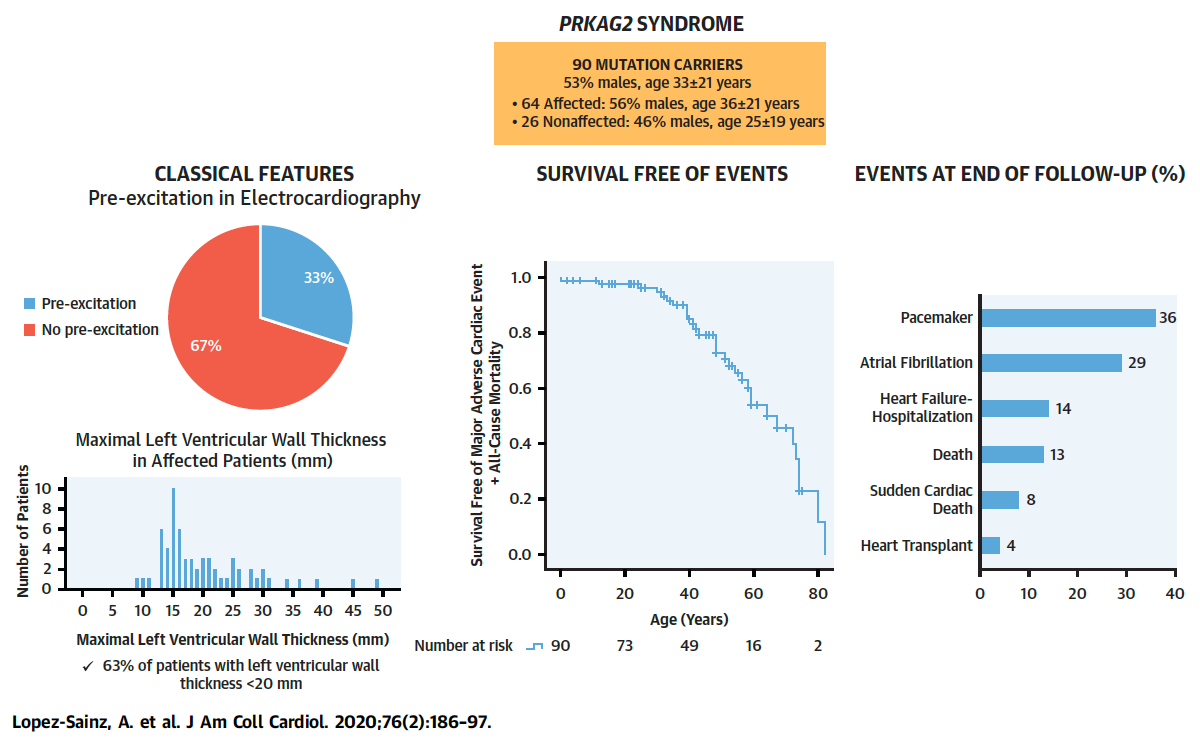
Figure 2. Manifestations, Survival Curve Free of Major Adverse Cardiac Events and Death, and
Outcomes in 90 Subjects With Variants in the PRKAG2 Gene. Full paper here.
We believe that advances in the knowledge of DCM genetics will follow a similar path to what has happened in other areas of medicine such as oncology or hematology. Thus, in the same way that happens currently with certain neoplasms, it is very likely that in the future, genetic features will be incorporated when offering the most appropriate treatments to patients according to their genetic characteristics (something that is already happening in some genes considered of “high arrhythmic risk”). To advance in this area is critical to understand at molecular level how mutations lead to phenotype and what is the contribution of the interplay between genetics and environmental factors. Our group has been collaborating with Dr Lara-Pezzi’s group at CNIC to study in depth one of the most devastating types of inherited cardiomyopathies, Arrhythmogenic Cardiomyopathy Type 5 caused by TMEM43 mutations. To do so a full set of studies from mice to humans have been undertaken (Padron-Barthe et al. Circulation 2019; Dominguez et al. Circ Heart Fail 2021 and Dominguez et al. Heart Rhythm 2021) and the focus know is to develop new theraoies to treat this devastating disease.
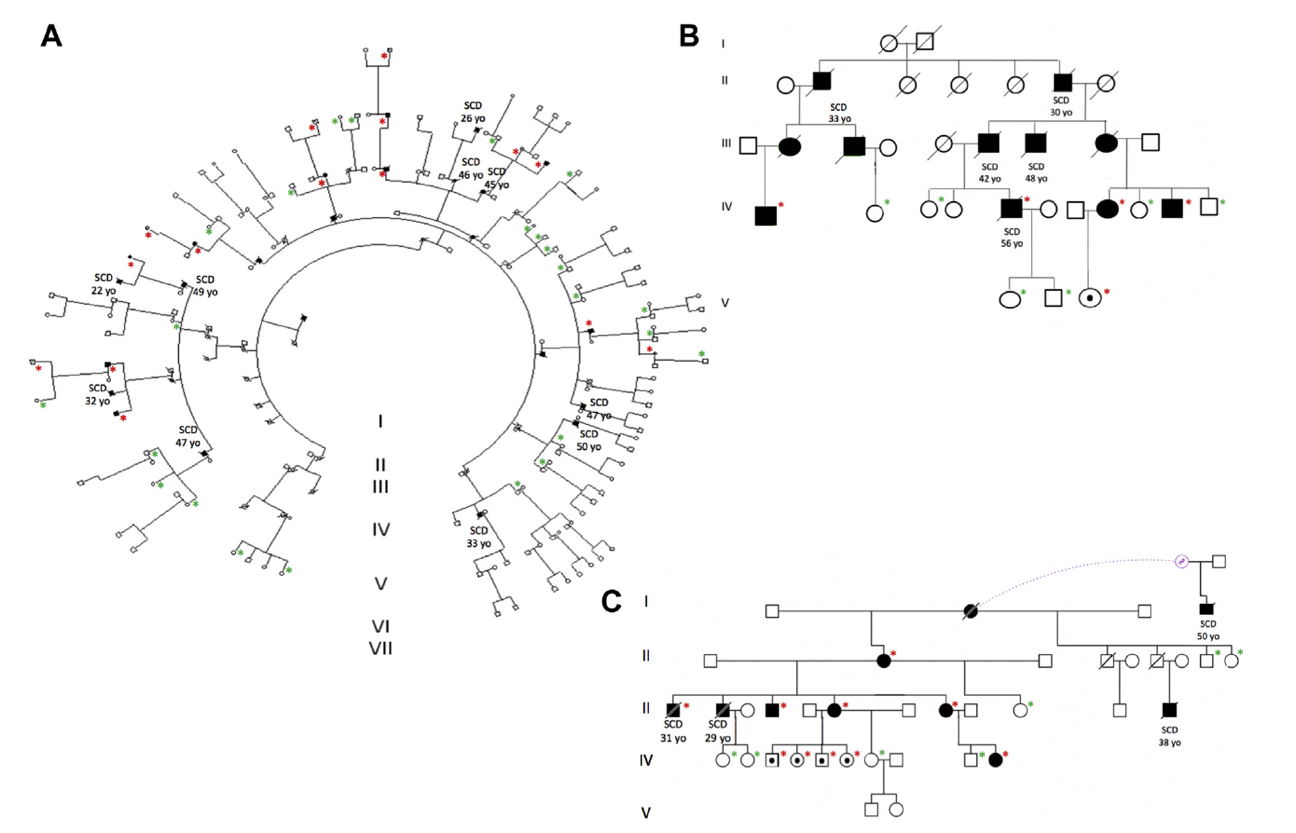
Figure 3. Pedigrees of 3 Spanish families with the p.S358L
mutation in TMEM43. Red asterisks represent confirmed genetic carriers and
green asterisks confirmed noncarriers. Full paper here.
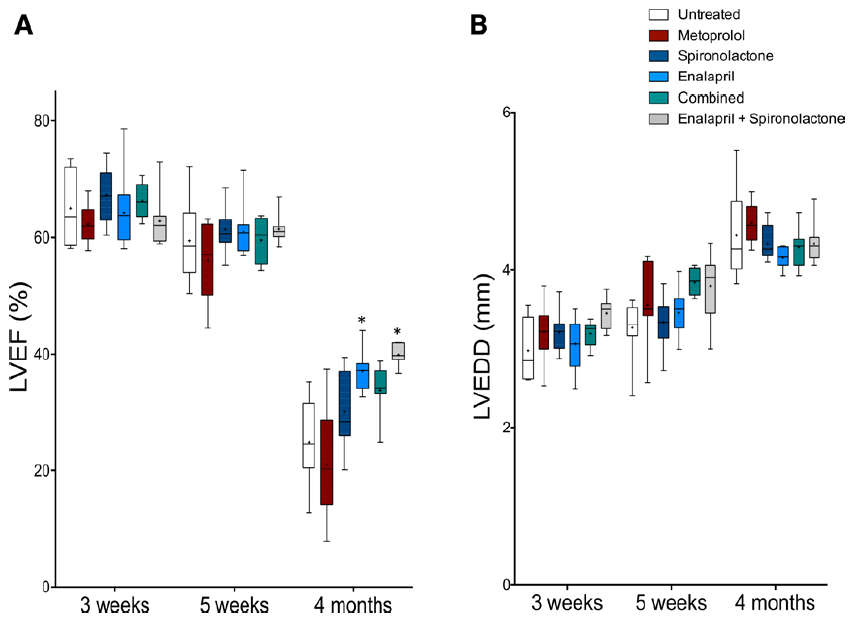
Figure 4. Enalapril improves cardiac function in arrhythmogenic right ventricular
cardiomyopathy type 5 (ARVC5) mice. ARVC5 mice were treated with different
heart failure (HF) drugs startingat 3 wk. Full paper here.
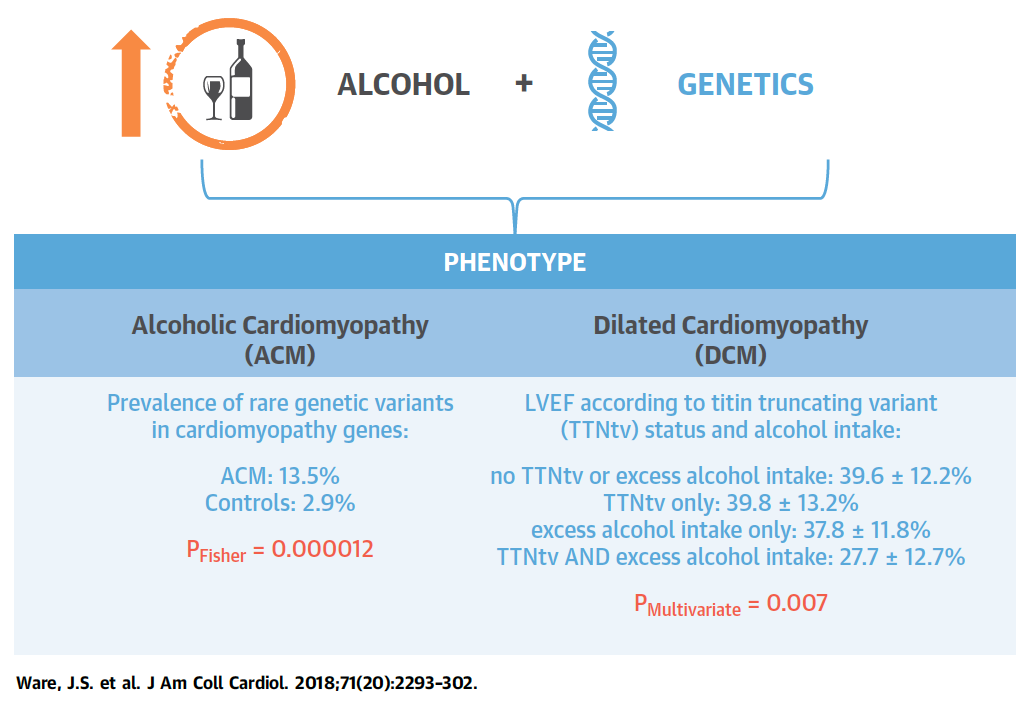
Figure 5. Alcohol Consumption and Genetic Background Act in
Concert to Determine Cardiac Phenotype. Full paper here.
In addition to guiding the management of the family by identifying carriers at risk, it is expected that in the very near future genetics will allow us to establish a prognosis and to provide specific individualized treatment in patients with DCM, which represents the bases of personalized medicine. As in all diseases with a hereditary component, genetic testing has become one of the cornerstones for the management of patients and families. In this lies the importance of identifying new causal genes and determining new mechanisms in other known genes to increase the diagnostic yield.
In summary, our group seeks to contribute to personalized medicine in cardiovascular field by fostering translational medicine in a group composed by cardiologists, geneticists, and basic researchers with expertise in animal models and molecular biology. We believe that a truly multidisciplinary team is the best way to answer complex questions and make a difference in the field!






