Angewandte Chemie International Edition: Controlling the speed of enzyme motors brings biomedical applications of nanorobots closer
A new study, published in the journal Angewandte Chemie International Edition, describes a tool for modulating nanomotors powered by enzymes, broadening their potential biomedical and environmental applications
A study by scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), the Universidad Complutense (UCM), Universidad de Girona (UdG), and the Institute for Bioengineering of Catalonia (IBEC), working together with other international centers, has overcome one of the key hurdles to the use of nanorobots powered by lipases, enzymes that play essential roles in digestion by breaking down fats in foods so that they can be absorbed.
The study was coordinated by Marco Filice of the CNIC Microscopy and Dynamic Imaging Unit—part of the ReDIB Infraestructura Científico Técnica Singular (ICTS)—, professor at Pharmacy Faculty (UCM) and ICREA Research Professor Samuel Sánchez of the IBEC. The article, published in the journal Angewandte Chemie International Edition, describes a tool for modulating motors powered by enzymes, broadening their potential biomedical and environmental applications.
Microorganisms are able to swim through complex environments, respond to their surroundings, and organize themselves autonomously. Inspired by these abilities, over the past 20 years scientists have managed to artificially replicate these tiny swimmers, first at the macro-micro scale and then at the nano scale, finding applications in environmental remediation and biomedicine.
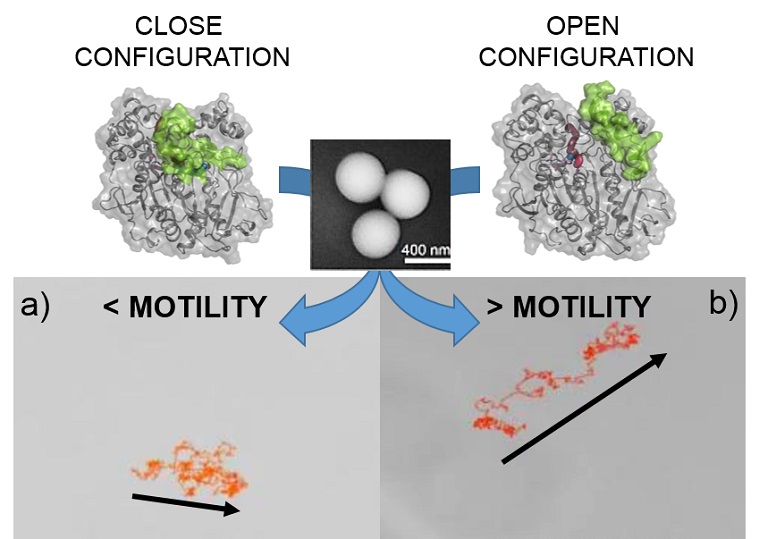
Lipases make excellent nanomotor components because their catalytic mechanism involves major conformational changes between an open, active form and a closed, inactive form
“The speed, load-bearing capacity, and ease of surface functionalization of micro and nanomotors has seen recent research advances convert these devices into promising instruments for solving many biomedical problems. However, a key challenge to the wider use of these nanorobots is choosing an appropriate motor to propel them,” explained Sánchez.
Over the past 5 years, the IBEC group has pioneered the use of enzymes to generate the propulsive force for nanomotors. “Bio-catalytic nanomotors use biological enzymes to convert chemical energy into mechanical force, and this approach has sparked great interest in the field, with urease, catalase, and glucose oxidase among the most frequent choices to power these tiny engines,” said Sánchez.
The CNIC group is a leader in the structural manipulation and immobilization of lipase enzymes on the surface of different nanomaterials. Lipases make excellent nanomotor components because their catalytic mechanism involves major conformational changes between an open, active form and a closed,
Recent advances in this field have made micro- and nanomotors promising devices for solving many biomedical problems
“In this project, we investigated the effect of modulating the catalytic activity of lipase enzymes to propel silicon-based nanoparticles,” explained Filice.
In addition to the 3-dimensional conformation of the enzyme, the team also investigated how controlling the orientation of the enzyme during its immobilization on the nanomotor surface affects its catalytic activity and therefore the propulsion of the nanorobots.
The researchers chemically modified the surface of silicon nanoparticles to generate three specific combinations of lipase conformations and orientations during immobilization: 1) open conformation plus controlled orientation; 2) closed conformation plus uncontrolled orientation; 3) a situation intermediate between 1 and 2.
The team analyzed the three types of nanorobot with spectroscopic techniques, assays to assess catalytic parameters related to enzyme activity, Dynamic Molecular simulations (performed by Professor Silvia Osuna’s team at UdG), and direct tracking of individual nanomotor trajectories by microscopy techniques. “The results demonstrate that combining an open enzyme conformation with a specific orientation on the nanomotor is critical to achieving controlled propulsion.”