Nature Cardiovascular Research: A CNIC team creates a dynamic 3D atlas of the formation of the embryonic heart
The 3D atlas has allowed the scientists to identify the beginning of left–right asymmetry in the heart
Scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) have used a collection of mouse tissue samples to create a 3D atlas of the formation of the heart during embryonic and fetal development. The 3D atlas has allowed the scientists to identify the first appearance of left–right asymmetry in the heart. The study, published today in Nature Cardiovascular Research, provides important information on the development of congenital heart malformations.
Study leader Dr Miguel Torres, whose lab specializes in the Genetic Control of Organ Development and Regeneration, said that the new study “will be of immense help to researchers seeking to understand heart development.”
A fundamental question in developmental biology is how the complex forms of tissues arise from simple geometric arrangements of cells, a process called morphogenesis. Investigating this question is relatively straightforward for accessible and easily visualized organs that develop according to a highly reproducible pattern, like the limbs or the eyes. But the task is more difficult in organs like the mammalian heart, which has a very varied morphology and is not easily accessed by videomicroscopy.
During the early phases of cardiogenesis, no two embryonic hearts are alike, and the differences can be so great that it can be difficult to decide which is at a more advanced stage of development. Nevertheless, assured Dr Torres, “the early, apparently divergent morphologies later converge to produce the well-formed, typical heart of the newborn animal.”

The challenge is to gauge the average progression of cardiac geometry from the wide variability encountered in nature and to learn to discriminate between the various physiological morphologies and anomalous forms. Doing this requires access to a sufficiently large sample collection.
“Only then will we be able to understand the properties of physiological morphogenesis and identify when and how anomolous forms arise,” explained Dr Torres.
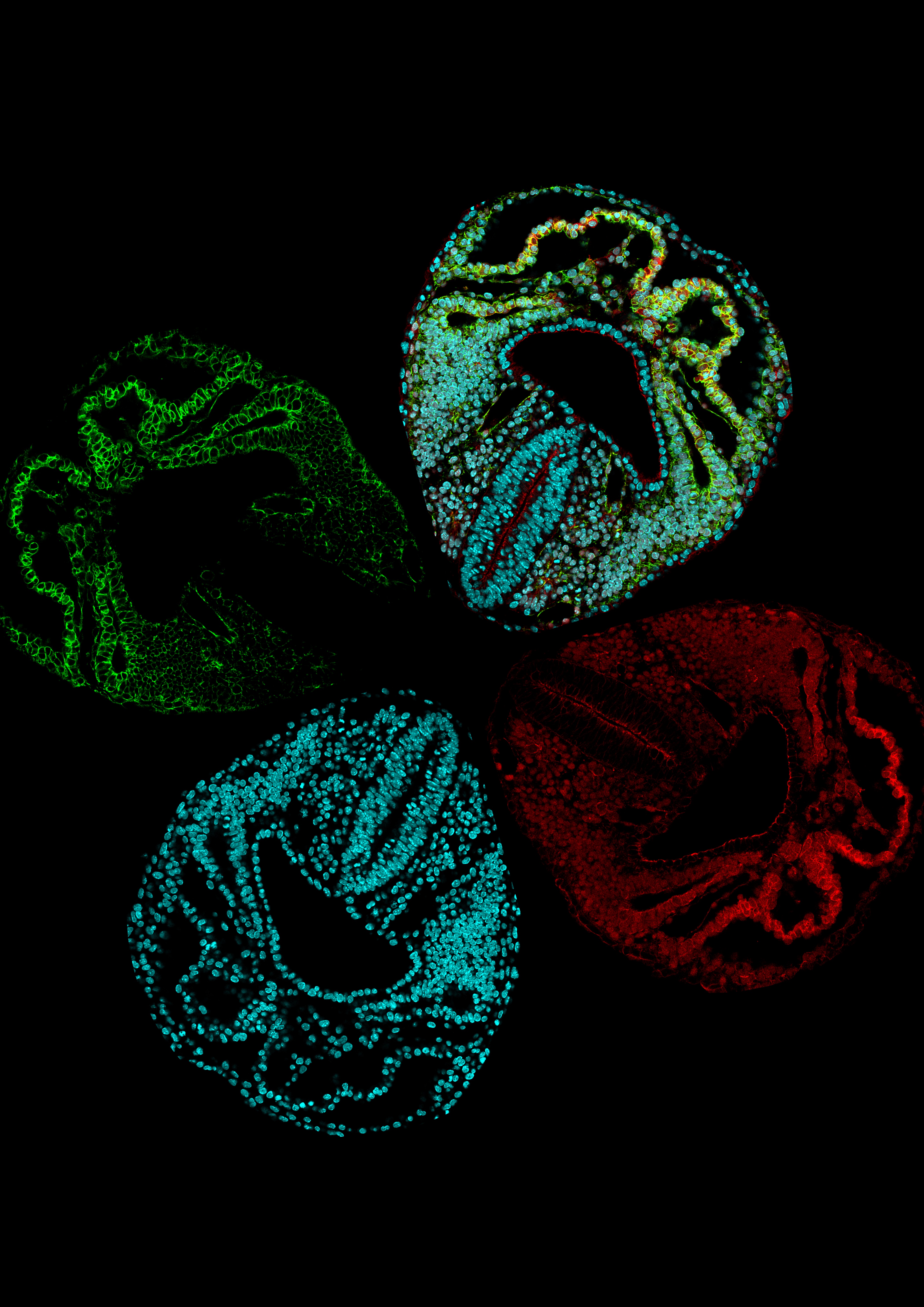
To get around the difficulties of obtaining live images, the CNIC team acquired a large collection of high resolution images of embryonic mouse hearts at key developmental stages and at a high temporal density.
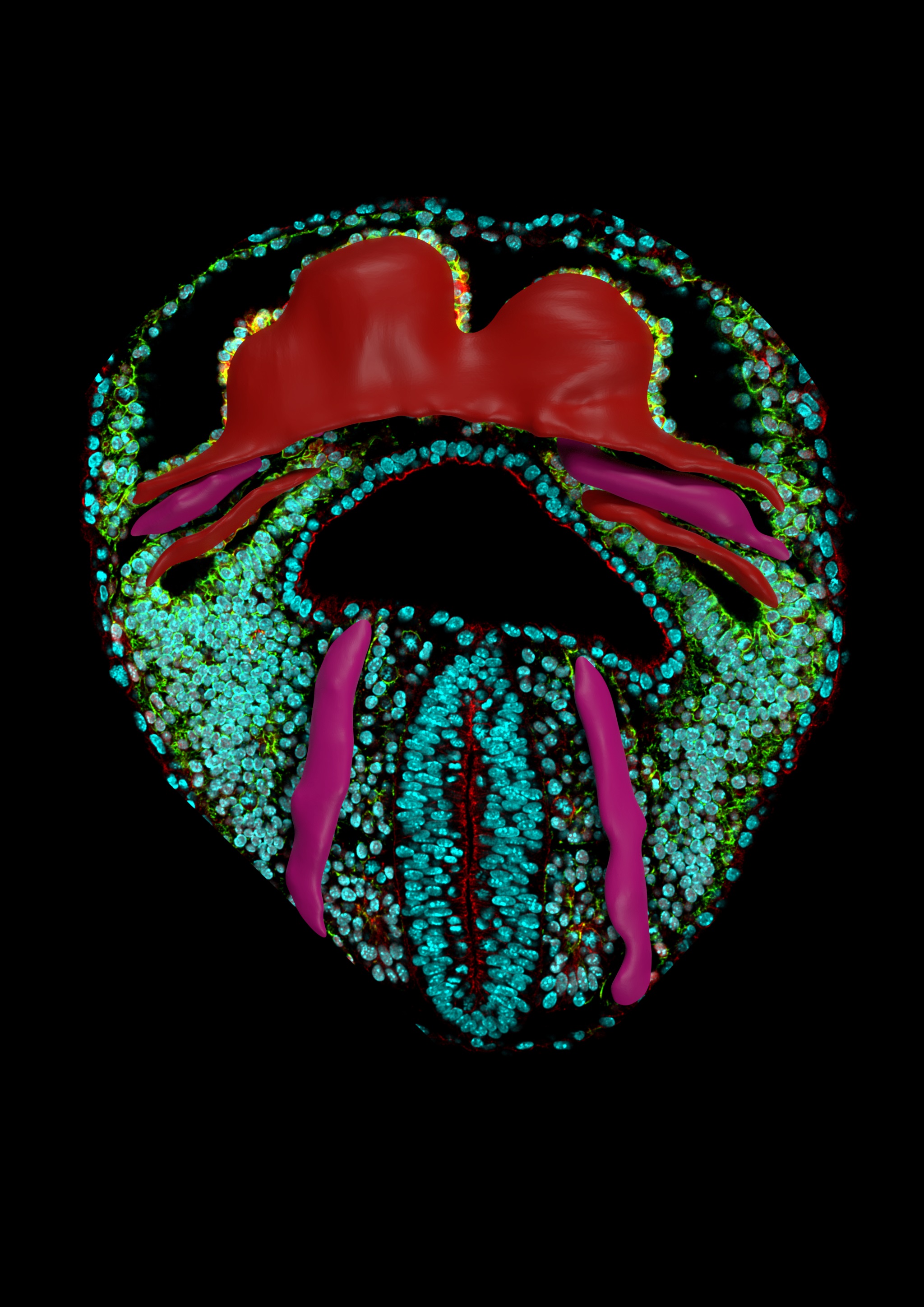
“We realized that we could not examine the morphogenesis of the heart in isolation from that of the adjoining tissues, because the formation of the heart tube can be likened to a geological fold, produced in a continuous layer of mesoderm (one of the cell layers in the ealy embryo) in the pericardiac cavity,” said Dr Torres. First author Isaac Estaban described how the team therefore “captured images of all the tissues in the pericardiac cavity, as well as the endoderm of the underlying anterior intestine.”
The investigators converted all the images into digital form and, using a morphometric staging system, ordered them into a temporal series, a superior approach to relying on the moment at which embryos were obtained, which does not necessarily correspond to the true timing of morphological development.
The CNIC scientists used a recently developed approach called mapping between surfaces. This technique generates corresponding surface point maps of similar structures at a high point density that allows reconstruction of the entire surface of the objects.
“This method allowed us to identify equivalent positions in groups of specimens from a similar developmental stage or at consecutive developmental stages,” said Esteban. Using this approach, the team created a temporal 3D atlas that shows the trajectory of the formation of the heart tube and reveals local morphological variability at each stage.
The atlas reveals that the regions usually involved in heart malformations are also the regions that show high morphological heterogeneity or variability during development. “This observation suggests that this morphological variability could underlie the high incidence of congenital heart malformations, which affect 1% of live newborn babies,” said Esteban.
The same approach used in this study can also be used to measure the moprphogenesis of any organ or organoid (an in vitro model of a developing organ).
Dr Torres remarked that, while a picture may be worth a thousand words, “a movie provides much more information than a thousand individual images,” and affirmed that “the dynamic 3D atlas and the morphometric staging system described in the study will be immensely useful and a source of inspiration for scientists interested in understanding the development of the heart.”
The main limitation of the study is that it reconstructs a dynamic process from fixed images, and so does not reveal details of the cellular basis of tissue deformation. Nevertheless, the atlas will be indespensible for efforts to define these cellular interactions, and scientists are already working to incorporate cell data into the new dynamic atlas of heart development.