ACS Nano: CNIC scientists describe a possible disease-causing mechanism in hypertrophic cardiomyopathy
A team led by Dr. Jorge Alegre-Cebollada has shown, for the first time, an association between hypertrophic cardiomyopathy and mechanical alterations to a component of the contractile machinery of the heart
Scientists at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) have described a potential disease-causing mechanism in hypertrophic cardiomyopathy (HCM), the most frequent hereditary disease of the heart. The study, published in the journal ACS Nano, provides the first description of an association between this disease and mechanical alterations to a component of the contractile machinery of the heart.
The heart muscle is under constant mechanical stress throughout life as it contracts to pump blood to the body. The laboratory led by Dr. Jorge Alegre-Cebollada investigates how the mechanical properties of the cardiac proteins determine the physiological behavior of this muscle and how alterations to these properties lead to the appearance of diseases like HCM. In this disease, the most frequent hereditary disease affecting the heart, the left ventricle becomes enlarged, and severe manifestations include heart failure and sudden death.
Scientists have known for more than 20 years that HCM is caused by mutations in proteins with a mechanical function in the heart. One of the challenges of cardiovascular genetics is to identify which among the genetic variants found in patients and their families cause disease. Knowing if a mutation is disease-causing or not is important because this information will determine the clinical follow-up of family members and, potentially, their treatment.
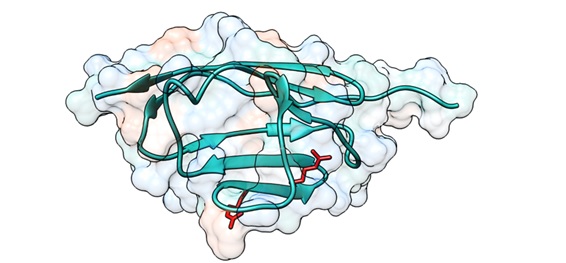
The new study, coordinated by Dr. Jorge Alegre-Cebollada, analyzed cardiac myosin-binding protein C (cMyBP-C).
First author Carmen Suay-Corredera explained that cMyBP-C, which regulates heart contraction, is the most frequently mutated protein in HCM patients. “A high proportion of mutations in the cMyBP-C gene cause amino-acid changes in the protein; however, the mechanisms by which these mutations cause HCM are not precisely known.”
Dr. Alegre-Cebollada’s group, in close partnership with clinical and molecular researchers in Europe and the US, set up a database of cMyBP-C variants with a clear link to HCM in order to define the molecular defects underlying the disease.
Using bioinformatics and experimental approaches, the research team discovered that around half of these mutations affect the integrity of cMyBP-C messenger RNA (mRNA) or protein. These results have already been accepted for publication in the Journal of Biological Chemistry and have been the subject of a commentary article in the leading medical genetics journal Genetics in Medicine.
While alterations to mRNA or protein integrity could explain the pathogenicity of half the mutations analyzed in the earlier study, Suay-Carredera pointed out that the other half do not cause disease via this route.
“It is precisely these variants, which cause HCM through unknown mechanisms, that we analyzed in the new study,” explained Dr. Alegre-Cebollada, who leads the Molecular Mechanics of the Cardiovascular System group at the CNIC.
Using advanced biophysical techniques based on atomic force microscopy, the team showed that some of the disease-causing mutations in cMyBP-C produce defects in the mechanical properties of the protein that can alter the contractile function of cardiomyocytes in HCM patients.
“We are now investigating the disease-causing mechanisms of those variants that have not been linked to any relevant alteration in previous studies,” said Dr. Alegre-Cebollada. For this project, the scientists are working with a range of experimental systems, from molecular systems to animal models of HCM.

Identifying the molecular mechanisms underlying HCM is essential for determining which cMyBP-C mutations cause the disease. This knowledge is therefore also crucial for the clinical follow-up and possible treatment of patients and their families, say the authors.
The study was funded by the Ministerio de Ciencia e Innovación, the European Research Area Network on Cardiovascular Diseases (MINOTAUR consortium, through the Instituto de Salud Carlos III), the Comunidad de Madrid, the US National Institutes of Health, the government of the Basque region, the Italian Ministry of Education, Universities and Research, and postdoctoral fellowships from the School of Medicine and the Maternal and Child Health Institute at Stanford University.
The research team also included scientists from the Cardiovascular Biomedical Research Network (CIBERCV).